Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Ewa Raczynska.
Prototropic tautomers always differ by the positions of labile proton(s) and π-electrons. The number of possible tautomeric forms is an internal property of the tautomeric molecule. It is a consequence of the number of labile protons and the number of conjugated tautomeric sites.
- tautomeric aromatic azines
- pyrimidine nucleic acid bases
- complete tautomeric mixtures
- internal effects
1. Introduction
Many organic π-electron heterosystems, including natural products, display a particular case of the constitutional isomerism of functional groups called prototropy. This structural phenomenon has been clearly explained, more than eighty years ago, by Pauling [1], who not only showed the fundamental relation between prototropy and resonance, but also indicated the important difference between tautomeric and resonance structures. According to his explanation, prototropic conversions are reversible processes that can run intra- or intermolecularly. During tautomerization, labile proton(s) can move between two or more conjugated functional groups together with the delocalization of π-electrons, leading to the mixture of two or more constitutional isomers, called tautomers.
Prototropic tautomers always differ by the positions of labile proton(s) and π-electrons [1,2][1][2]. The number of possible tautomeric forms is an internal property of the tautomeric molecule. It is a consequence of the number of labile protons and the number of conjugated tautomeric sites. Although the most favored tautomer is very often selected to determine the name and formula of the tautomeric compound, which cannot be identified only with one Lewis structure. Each tautomeric derivative can be described by means of two (or more) structures (tautomers) being in equilibrium, whereas electron delocalization in each tautomer can be described by the corresponding resonance hybrid. For a single tautomer, the number of possible resonance structures results from the position of labile protons and double bonds. A different situation takes place for the relative stabilities of individual tautomers. They strongly depend on various internal and external factors that affect tautomeric preferences. Among the internal factors, the polarity, resonance stability (aromaticity), acidity–basicity of conjugated tautomeric sites, stability of functional groups, and substituents effects, as well as intramolecular interactions, play a particular role. For the external factors, usually, the solvent, pH, excess electron(s), other molecules, ions, radicals, oxidizing or reducing agents, ultraviolet (UV), and γ- and X-ray are considered.
The Pauling explanation of the prototropy phenomenon [1] has been employed in the IUPAC definition of prototropic tautomerism (IUPAC—International Union on Pure and Applied Chemistry) [3]. Only proton-transfers accompanied by the migration of double bonds refer to prototropic conversions in the tautomeric molecule. In other words, prototropic rearrangements always run in relation with electron delocalization [1,2,3][1][2][3]. The labile proton(s) can move from proton-donor site(s) to proton-acceptor site(s) separated by different conjugated spacers according to 1,3-, 1,5-, 1,7-, 1,9-proton shift, etc. Other intramolecular transfer(s) of H+ or H● leading to a separation of positive and negative charges or to a separation of paired electrons cannot be considered as prototropy, and, consequently, zwitterions or polyvalent radicals formed in these processes cannot be classified as prototropic tautomers.
Prototropic conversions in aromatic heterocompounds, including nucleic acid bases, have been reviewed by Katritzky (died in 2014) and his co-workers in the 1960–2010 period (see, for example, refs. [4,5][4][5]). They compiled experimental and computational results mainly for favored tautomers (percentage contents > 1%), and considered most minor (<1%) and all rare tautomers (<0.01%) as negligible in tautomeric mixtures. This kind of treatment of tautomeric systems has led to some discrepancies in the literature, particularly for ionized, protonated, and deprotonated forms, for which prototropy has been usually neglected. Experimental and/or theoretical investigations have been carried out for tautomers that are favored in neutral isomeric mixtures. In the case of pyrimidine nucleic acid bases, the canonical forms or their major tautomers (two or three structures) have been the most frequently considered. These kinds of investigations for the selected isomers are usually partial.
2. Principles of Prototropic Equilibria
Four types of prototropic conversions {keto-enol, imine-enamine, imine-amine (amidine), and/or amide-iminol} can be distinguished for pyrimidine bases, uracil (U), thymine (T), cytosine(C), isocytosine {iC—structural part of guanine (G)}, and 4-aminopyrimidine {4APM—structural part of adenine (A)}, as well as for bicyclic purine bases, G and A, and for their metabolites such as hypoxanthine (HX), xanthine (X), and uric acid (UA). These equilibria are summarized in Table 1 for selected tautomeric moieties. For all of them, the labile proton can move between the conjugated sites according to the analogous scheme of reversible inter- or intramolecular rearrangement accompanied by the migration of the corresponding π-electrons [1,2,3][1][2][3].Table 1.
Prototropic conversions occurring for nucleic acid bases in selected tautomeric fragments.
| Equilibria | Name of Conversion |
|---|---|
| >CH–C(=O)– ⇌⇌ >C=C(–OH)– | keto-enol |
| >CH–C(=N–)– ⇌⇌ >C=C(–NH–)– | imine-enamine |
| –NH–C(=N–)– ⇌⇌ –N=C(–NH–)– | imine-amine (amidine) |
| –NH–C(=O)– ⇌⇌ –N=C(–OH)– | amide-iminol |
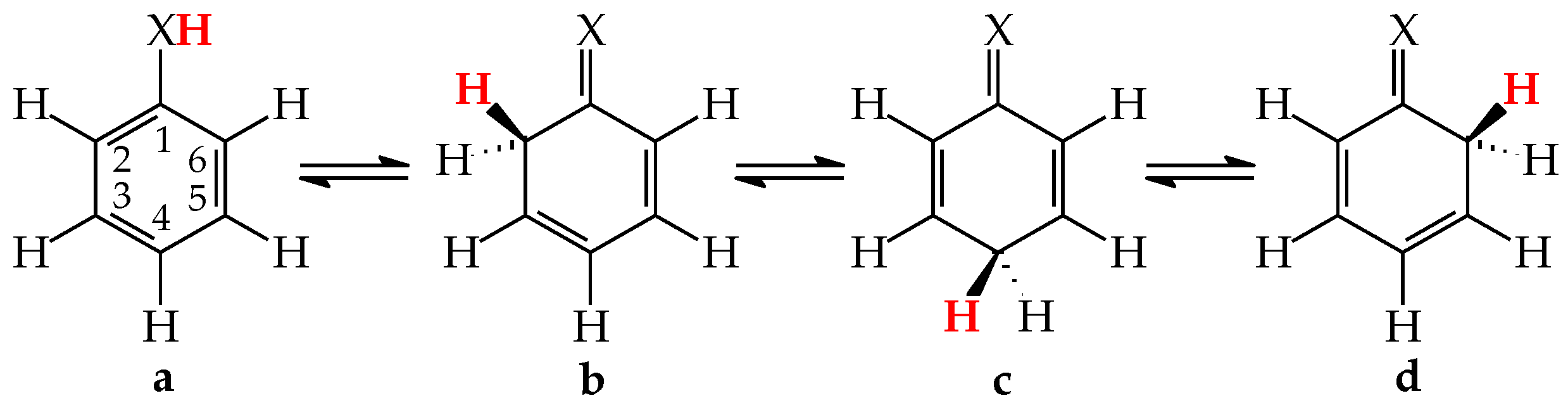
Figure 1.
Prototropic equilibria in phenol (X = O) and aniline (X = NH). The labile proton is indicated in bold red color.
References
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: New York, NY, USA, 1960.
- Raczyńska, E.D.; Kosińska, W.; Ośmiałowski, B.; Gawinecki, R. Tautomeric Equilibria in Relation to pi-Electron Delocalization. Chem. Rev. 2005, 105, 3561–3612.
- Perrin, C.L.; Agranat, I.; Bagno, A.; Braslavsky, S.E.; Fernandes, P.A.; Gal, J.-F.; Lloyd-Jones, G.C.; Mayr, H.; Murdoch, J.R.; Nudelman, N.S.; et al. Glossary of Terms Used in Physical Organic Chemistry (IUPAC Recommendations 2021). Pure Appl. Chem. 2022, 94, 353–534.
- Elguero, J.; Marzin, C.; Katritzky, A.R.; Linda, P. The Tautomerism of Heterocycles (Advances in Heterocyclic Chemistry: Supplement 1). Academic Press: New York, NY, USA, 1976.
- Stanovnik, B.; Tišler, M.; Katritzky, A.R.; Denisko, O.V. The Tautomerism of Heterocycles: Substituent Tautomerism of Six-Membered Ring Heterocycles. Adv. Heterocyclic Chem. 2006, 91, 1–134.
- Rappoport, Z. (Ed.) The Chemistry of Enols; Wiley: Chichester, UK, 1990.
- Perez, P.; Toro-Labbe, A. Characterization of Keto-Enol Tautomerism of Acetyl Derivatives from the Analysis of Energy, Chemical Potential, and Hardness. J. Phys. Chem. A 2000, 104, 1557–1562.
- Hansen, P.E. Structural Studies of β-Diketones and Their Implications on Biological Effects. Pharmaceuticals 2021, 14, 1189.
- Reichardt, C. Solvent and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2002.
- Lammertsma, K.; Prasad, B.V. Imine-Enamine Tautomerism. J. Am. Chem. Soc. 1994, 116, 642–650.
- Fogarasi, G. Studies on Tautomerism: Benchmark Quantum Chemical Calculations on Formamide and Formamidine. J. Mol. Struct. 2010, 978, 257–262.
- Rappoport, Z. (Ed.) The Chemistry of Phenols; John Wiley & Sons: Chichester, UK, 2003.
- Cook, M.J.; Katritzky, A.R.; Linda, P.; Tack, R.D. Aromatic Resonance Energies from Equilibrium Data. Tetrahedron Lett. 1972, 49, 5019–5022.
- Capponi, M.; Gut, I.G.; Hellrung, B.; Persy, G.; Wirz, J. Ketonization Equilibria of Phenol in Aqueous Solution. Can. J. Chem. 1999, 77, 605–613.
- Zhu, L.; Bozzelli, J.W. Kinetcs and Thermochemistry for the Gas-Phase Keto-Enol Tautomerism of Phenol↔2,4-Cyclohexadienone. J. Phys. Chem. A 2003, 107, 3696–3703.
- Ośmiałowski, B.; Raczyńska, E.D.; Krygowski, T.M. Tautomeric Equilibria and pi Electron Delocalization for some Monohydroxyarenes—Quantum Chemical Studies. J. Org. Chem. 2006, 71, 3727–3736.
- Gomez, I.; Rodriguez, E.; Reguero, M. New Insights into the Interconversion Mechanism between Phenol and Its Isomers. J. Mol. Struct. (Theochem) 2006, 767, 11–18.
- Raczyńska, E.D.; Kolczyńska, K.; Stępniewski, T.M. Tautomeric Preferences and π-Electron Delocalization for Redox Forms of Phenol. Comput. Theoret. Chem. 2011, 963, 176–184.
- Zeh, D.; Bast, M.; Rap, D.B.; Schmid, P.C.; Thorwith, S.; Brünken, S.; Schlemmer, S.; Schäfer, M. Cryogenic Messenger-IR Ion Spectroscopy Study of Phenol & Aniline Molecular Ions and the Common Fragment Ion + Formed by EI-MS. J. Mol. Spectrosc. 2021, 378, 111453.
- Korth, H.-G.; Mulder, P. Anthrone and Related Hydroxyarenes: Tautomerization and Hydrogen Bonding. J. Org. Chem. 2013, 78, 7674–7682.
- Raczyńska, E.D. Quantum-Chemical Search for Keto Tautomers of Azulenols in Vacuo and Aqueous Solution. Symmetry 2021, 13, 497.
- Raczyńska, E.D.; Stępniewski, T.M.; Kolczyńska, K. Consequence of One-Electron Oxidation and One-Electron Reduction for Aniline. J. Mol. Model. 2011, 17, 3229–3239.
- Walker, S.W.C.; Mark, A.; Verbuyst, B.; Bogdanov, B.; Campbell, J.L.; Hopkins, W.S. Characterizing the Tautomers of Protonated Aniline Using Differential Mobility Spectrometry and Mass Spectrometry. J. Phys. Chem. A 2018, 122, 3858–3865.
- Kune, C.; Delvaux, C.; Haler, J.R.N.; Quinton, L.; Eppe, G.; De Pauw, E.; Far, J. A Mechanistic Study of Protonated Aniline to Protonated Phenol Substitution Considering Tautomerization by Ion Mobility Mass Spectrometry and Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 2238–2249.
- Lichte, D.; Pirkl, N.; Heinrich, G.; Goebel, J.F.; Koley, D.; Gooβen, L.J. Palladium-Catalyzed para-C−H Arylation of Anilines with Aromatic Halides. Angew. Chem. Int. Ed. Engl. 2022, 61, e202210009.
- Naylor, C.N.; Schaefer, C.; Kirk, A.; Zimmermann, S. The Origin of Izomerization of Aniline Revealed by High Kinetic Energy Ion Mobility Spectrometry (HiKE-IMS). Phys. Chem. Chem. Phys. 2023, 25, 1139.
- Schleyer, P.V.R.; Pühlhofer, F. Recommendations for the Evaluation of Aromatic Stabilization Energies. Org. Lett. 2002, 4, 2873–2876.
- Raczyńska, E.D.; Juras, W. Effects of Ionization and Proton Transfer on Bond Length Alternation in Favored and rare isomers of Isocytosine. Comput. Theoret. Chem. 2019, 1148, 16–26.
- Raczyńska, E.D.; Kamińska, B. Prototropy and π-Electron Delocalization for Purine and Its Radical Ions—DFT Studies. J. Phys. Org. Chem. 2010, 23, 828–835.
- Raczyńska, E.D.; Gal, J.-F.; Maria, P.-C.; Kamińska, B.; Igielska, M.; Kurpiewski, J.; Juras, W. Purine Tautomeric-Preferences and Bond-Length Alternation in Relation with Protonation-Deprotonation and Alkali-Metal Cationization. J. Mol. Model. 2020, 26, 93.
- Raczyńska, E.D.; Makowski, M.; Zientara-Rytter, K.; Kolczyńska, K.; Stępniewski, T.M.; Hallmann, M. Quantum-Chemical Studies on the Favored and Rare Tautomers of Neutral and Redox Adenine. J. Phys. Chem. A 2013, 117, 1548–1559.
- Raczyńska, E.D.; Kamińska, B. Structural and Thermochemical Consequences of Prototropy and Ionization for the Biomolecule Xanthine in Vacuo. J. Chem. Thermodyn. 2022, 171, 106788.
- Szeląg, M.; Raczyńska, E.D. Tautomeric Equilibria for Uric Acid. Trends Org. Chem. 2008, 12, 19–31.
- Raczyńska, E.D.; Makowski, M.; Szeląg, M.; Kamińska, B.; Zientara, K. Importance of CH Tautomers in the Tautomeric Mixture of Uric Acid. J. Mol. Struct. (Theochem) 2010, 947, 83–91.
- Raczyńska, E.D.; Makowski, M.; Hallmann, M.; Kamińska, B. Geometric and Energetic Consequences of Prototropy for Adenine and Its Structural Models—A Review. RSC Adv. 2005, 5, 36587.
More
