Recent scientific developments in understanding the lifespan of the corpus luteum provided new insights into dynamic molecular changes occurring during transition of this fascinating endocrine gland into the organ supporting pregnancy. Processes such as oocyte-sperm interaction, preparation of the uterus for implantation, blastocyst attachment, and successful gestation are mainly driven by progesterone, a steroid hormone produced by the corpus luteum. Inadequate production of progesterone has been described in several mammals, including humans, as luteal phase deficiency, a condition in which endogenous progesterone is insufficient to support pregnancy. Thus, it is essential to extend knowledge about the molecular mechanisms controlling the function of the corpus luteum. Unfortunately, still there is a lack of data explaining the regulation of core molecules responsible for the maintenance of luteal function. Recent studies shed a new light on the molecular mechanisms supporting luteal function, involving microRNAs.
- corpus luteum
- microRNA
- progesterone
- estradiol
- NR4A1
- pregnancy
- corpus luteum regression
- corpus luteum maintenance
1. Introduction
The fundamental role of miRNAs in ovarian function was proved by knockout of DICER, which inhibited follicle growth, reduced the ovulation rate and led to faulty oocyte development [[1][2][3]]. Further studies showed a number of differentially expressed miRNAs in the bovine and ovine corpus luteum (CL) during development, regression, or its rescue during pregnancy [[4][5][6][7][8][9]]. Interestingly, a greater amount of miRNAs was found in the mature than in the developing CL, indicating their potential role in the maintenance of luteal function. Nevertheless, not many studies have provided information on the roles of specific miRNA–mRNA interactions on the function of luteal cells, while only a few have shown changes in the expression of miRNAs occurring in the CL during early pregnancy [[10][7]].
Recently, 14 differentially expressed miRNAs were identified in porcine CL collected right after maternal recognition of pregnancy (day 14 of pregnancy) and luteolysis (day 14 of the estrous cycle) [[10]]. Overall, seven miRNAs were upregulated (e.g., miR-21a-3p, miR-345-3p, miR-371-5p), and seven miRNAs were downregulated (e.g., miR-181a, miR-532-3p, miR-99b) in the porcine CL on day 14 of the estrous cycle compared to the corresponding day of pregnancy. Interestingly, among miRNA targets upregulated during pregnancy, genes encoding known regulators of luteolysis were found (e.g., EDN1, FOS, JUN, PTGS2, ESR2) [[11][12][13][14]], while miRNAs elevated in the CL during luteolysis could target genes associated with luteal function maintenance (e.g., PGR, VEGFR1, CREB, PTGER2). Further in silico analysis suggested that miRNAs highly expressed in the CL during early pregnancy can be involved in the cell cycle, cell death and survival, cellular development, growth, and proliferation—events characterizing the time when a decision is made either to regress or maintain the luteal function. Notably, the expression of miRNAs was examined in the whole luteal tissue. Therefore, the aforementioned biological processes can be potentially regulated by differentially expressed miRNAs in various cells forming the porcine CL, such as the endothelial cells, immune cells, or fibroblasts, and not only the luteal cells. Other studies performed on the bovine CL identified 15 miRNAs differentially expressed on day 18 of pregnancy vs. the corresponding day of the estrous cycle, while among predicted targets of these miRNAs, genes involved in immune-related events and apoptosis were found [[7]].
Previously, the high expression of two clusters, miR-183-96-182 and miR-212-132, was noticed in the luteal vs. follicular tissue of cows, while miR-96 was found to be an important regulator of steroidogenesis and cell survival [[9]]. In pigs, miRNAs upregulated in the CL of pregnant animals belong to three independent clusters: miR-99b, miR-532, and miR-181a [[10]]. Again, among targets of miRNAs occurring in the same cluster (i.e., miR-532 and miR-99b), crucial regulators of luteolysis were found, including, for example, EDN1, FOS, JUN, NR4A1, OXTR, and PTGS2 [[11][12][13][14]]. Interestingly, identified clusters are conserved or broadly conserved among different animal species and humans; thus, it is possible that some of them can support luteal function in other species as well, especially since miRNAs belonging to cluster miR-99b were previously detected among mostly abundant miRNAs in the mature bovine CL [[8]].
2. Influence and application
Luteotrophic and/or luteolytic factors were suggested to be effective modulators of miRNA expression [[9][15]]. In pigs, incubation of luteal tissue slices with estradiol-17beta (E2) stimulated the expression of miRNAs belonging to the miR-99b cluster [[10]]. Further, bioinformatics analysis showed multiple SP1 and estrogen receptor 1 (ESR1) binding sites in the promoter of the miR-99b cluster. SP1 is a zinc finger transcription factor that acts synergistically with ESR1, one of the genomic receptors of estradiol, in the regulation of gene transcription. Therefore, we suggest that E2 increases the expression of miR-99b by the ESR1-SP1 pathway in the CL during early pregnancy. Remarkably, the aforementioned effects of E2 on the expression of the miRNA-99b cluster were concomitant with the inhibitory effect of E2 on the expression of NR4A1 and AKR1C1 genes in luteal tissue slices [[10]]. NR4A1 is encoded by immediate-early response genes, which can induce apoptosis or elevate transcription of AKR1C1, an enzyme responsible for the metabolism of progesterone [[16][17]]. In addition, NR4A1 was identified as a marker of luteolysis in the CL of cows and rats [[16][17][18]]. Our further studies showed that the transfection of luteal tissue slices with mimics of miRNAs belonging to the miR-99b cluster decreases the expression of NR4A1 and AKR1C1 genes, and increases the production of progesterone by luteal tissue slices of pigs [[10]]. Therefore, it seems likely that E2 can induce the expression of the miR-99b cluster in porcine CL during the peri-implantation period, leading to decreased expression of genes involved in luteolysis (Figure 1).
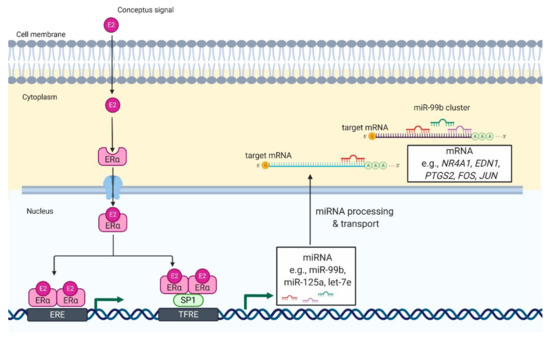
Figure 1. Proposed model of E2-mediated regulation of miRNA expression in luteal tissue during early pregnancy in pigs. E2 enters the luteal cell and binds with its genomic receptor, ERα, located in the cytoplasm. It leads to formation of ERα homodimers and their translocation to the nucleus. In the nucleus, ERα homodimers bind to (1) an estrogen-responsive element (ERE) or (2) transcription factor SP1, which binds to transcriptional factor responsive elements (TFRE) in the promoter region of miR-99b cluster. As a result, there is an increase in the expression of miRNAs belonging to miR-99b cluster (miR-99b, miR-125a, and let-7e), which can target mRNA involved in luteolysis including NR4A1, nuclear receptor subfamily 4 group A member 1; EDN1, endothelin 1; FOS, fos proto-oncogene; JUN, jun proto-oncogene. That mechanisms support the function of luteal cells and allow continuation of progesterone production required for pregnancy establishment and maintenance.
References
- Xiaoman Hong; Lacey J. Luense; Lynda K. McGinnis; Warren B. Nothnick; Lane Christenson; Dicer1 is essential for female fertility and normal development of the female reproductive system.. Endocrinology 2008, 149, 6207-6212, 10.1210/en.2008-0294.
- Fuchou Tang; Masahiro Kaneda; Dónal O’Carroll; Petra Hajkova; Sheila C. Barton; Y. Andrew Sun; Caroline Lee; Alexander Tarakhovsky; Kaiqin Lao; Azim Surani; Maternal microRNAs are essential for mouse zygotic development. Genome Research 2007, 21, 644-648, 10.1101/gad.418707.
- Ankur K. Nagaraja; Claudia Andreu-Vieyra; Heather L. Franco; Lang Ma; Ruihong Chen; Derek Y. Han; Huifeng Zhu; Julio E. Agno; Preethi H. Gunaratne; Francesco John DeMayo; Martin M Matzuk; Deletion of Dicer in somatic cells of the female reproductive tract causes sterility.. Molecular Endocrinology 2008, 22, 2336-52, 10.1210/me.2008-0142.
- Stephanie D. Fiedler; Martha Z. Carletti; Xiaoman Hong; Lane Christenson; Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells.. Biology of Reproduction 2008, 79, 1030-7, 10.1095/biolreprod.108.069690.
- D. McBride; W. Carré; S. D. Sontakke; C. O. Hogg; A. Law; Francesc X. Donadeu; Michael Clinton; Identification of miRNAs associated with the follicular–luteal transition in the ruminant ovary. Reproduction 2012, 144, 221-233, 10.1530/rep-12-0025.
- Tenghe Ma; Hao Jiang; Yan Gao; Yumin Zhao; Lisheng Dai; Qiuhong Xiong; Yanli Xu; Zhihui Zhao; Jiabao Zhang; Microarray analysis of differentially expressed microRNAs in non-regressed and regressed bovine corpus luteum tissue; microRNA-378 may suppress luteal cell apoptosis by targeting the interferon gamma receptor 1 gene. Journal of Applied Genetics 2011, 52, 481-486, 10.1007/s13353-011-0055-z.
- Samar W. Maalouf; Wan-Sheng Liu; Istvan Albert; Joy L. Pate; Regulating life or death: Potential role of microRNA in rescue of the corpus luteum. Molecular and Cellular Endocrinology 2014, 398, 78-88, 10.1016/j.mce.2014.10.005.
- Rreze M. Gecaj; Corina I. Schanzenbach; Benedikt Kirchner; Michael Pfaffl; Irmgard Riedmaier; Ry Y. Tweedie-Cullen; Bajram Berisha; The Dynamics of microRNA Transcriptome in Bovine Corpus Luteum during Its Formation, Function, and Regression. Frontiers in Genetics 2017, 8, , 10.3389/fgene.2017.00213.
- Bushra T Mohammed; Sadanand D. Sontakke; Jason Ioannidis; W. Colin Duncan; F. Xavier Donadeu; The Adequate Corpus Luteum: miR-96 Promotes Luteal Cell Survival and Progesterone Production. The Journal of Clinical Endocrinology & Metabolism 2017, 102, 2188-2198, 10.1210/jc.2017-00259.
- Emilia Przygrodzka; Gabriela Sokołowska; Kamil Myszczyński; Kamil Krawczynski; Monika M. Kaczmarek; Clustered microRNAs: The molecular mechanism supporting the maintenance of luteal function during early pregnancy. The FASEB Journal 2020, none, none, 10.1096/fj.201903007rr.
- Francisco J. Diaz; Milo C. Wiltbank; Acquisition of luteolytic capacity involves differential regulation by prostaglandin F2α of genes involved in progesterone biosynthesis in the porcine corpus luteum. Domestic Animal Endocrinology 2005, 28, 172-189, 10.1016/j.domaniend.2004.08.002.
- Francisco J. Diaz; Wenxiang Luo; M.C. Wiltbank; Effect of decreasing intraluteal progesterone on sensitivity of the early porcine corpus luteum to the luteolytic actions of prostaglandin F2alpha.. Biology of Reproduction 2010, 84, 26-33, 10.1095/biolreprod.110.084368.
- Emilia Przygrodzka; Krzysztof J. Witek; Monika M. Kaczmarek; A Andronowska; Adam J. Ziecik; Expression of factors associated with apoptosis in the porcine corpus luteum throughout the luteal phase of the estrous cycle and early pregnancy: Their possible involvement in acquisition of luteolytic sensitivity. Theriogenology 2015, 83, 535-545, 10.1016/j.theriogenology.2014.10.016.
- Emilia Przygrodzka; Monika M. Kaczmarek; Piotr Kaczyński; Adam J. Ziecik; P. Kaczy Ski; Steroid hormones, prostanoids, and angiogenic systems during rescue of the corpus luteum in pigs. Reproduction 2016, 151, 135-147, 10.1530/rep-15-0332.
- Samar W. Maalouf; Courtney L. Smith; Joy L. Pate; Changes in MicroRNA Expression During Maturation of the Bovine Corpus Luteum: Regulation of Luteal Cell Proliferation and Function by MicroRNA-34a1. Biology of Reproduction 2016, 94, 71, 10.1095/biolreprod.115.135053.
- Stocco, C.O.; Zhong, L.; Sugimoto, Y.; Ichikawa, A.; Lau, L.F.; Gibori, G. Prostaglandin F2alpha-induced expression of 20alpha-hydroxysteroid dehydrogenase involves the transcription factor NUR77. J. Biol. Chem. 2000, 275, 37202–37211. [Google Scholar] [CrossRef]
- Stocco, C.O.; Lau, L.F.; Gibori, G. A calcium/calmodulin-dependent activation of ERK1/2 614 mediates JunD phosphorylation and induction of nur77 and 20alpha-hsd genes by prostaglandin F2alpha in 615 ovarian cells. J. Biol. Chem. 2002, 277, 3293–3302. [Google Scholar] [CrossRef]
- Mehmet O. Atli; Robb W. Bender; Vatsal Mehta; Michele R. Bastos; Wenxiang Luo; Chad M Vezina; M.C. Wiltbank; Patterns of Gene Expression in the Bovine Corpus Luteum Following Repeated Intrauterine Infusions of Low Doses of Prostaglandin F2alpha1. Biology of Reproduction 2012, 86, 130, 10.1095/biolreprod.111.094870.
