Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Ajay Mittal.
Chitooligosaccharide (CHOS), a depolymerized chitosan, can be prepared via physical, chemical, and enzymatic hydrolysis, or a combination of these techniques.
- chitooligosaccharide
- derivatives
- foods
- additives
1. Introduction
Annually, crustacean processing wastes are generated at 6–8 metric tons (MT) across the globe, and approximately 1.5 MT was reported in Southeast Asia [1]. The biowaste generated during shrimp processing is unavoidable, and disposal has become a major problem for processing industries along with the increased expenditure. It can produce an adverse effect on the environment (i.e., pollution), which creates risks to human health when the disposal management of waste is carried out improperly. Nevertheless, these biowastes contain several valuable compounds when appropriate processing or technology is implemented to earn better profit via their valorization. Shrimp processing waste contains a wide range of valuable bioactive compounds such as astaxanthin, chitin, bioactive peptides, fatty acids, amino acids, etc. [2]. Also, shrimp shell is cardinal waste from shrimp processing industries. It contains a high amount of chitin, which can be converted to its derivatives, especially chitosan (CS) and CS can be further converted to chitooligosaccharide (CHOS) [3].
CHOS (β-1-4-linked d-glucosamine) is a water-soluble depolymerized product of CS extracted from shrimp shells and other crustacean wastes [4,5,6][4][5][6]. It is positively charged cationic oligosaccharide with an average molecular weight (MW) and degree of polymerization (DP) of less than 3.9 kDa and 20, respectively. Sometimes, much larger molecules (up to 20 kDa) are also called CHOS. Their superior characteristics, such as a low molecular weight, low polymerization degree, and high-water solubility, compared to those of chitin and CS make them more applicable. Various methods including physical, chemical, and enzymatic methods have been employed for CS hydrolysis by cleaving glycosidic bonds to produce CHOS [7]. In terms of their applications, CHOS has been widely used as antioxidant, antibacterial, and antifungal agents [8,9][8][9] and vectors in gene therapy [10]. The physical and biological activities of CHOS are governed primarily by the DP, MW, and DD.
CHOS possesses the reactive amino group at C-2 and hydroxyl groups at C-3 and C-6, which could favor chemical modification such as acylation, oxidation, etherification, graft copolymerization, cationization, phosphorylation, etc. [11], thus enhancing CHOS’ bioactivities. CHOS derivatives have been used to alleviate metastasis and tumor growth [12,13][12][13] and increase bone strength by preventing osteoporosis [14,15,16][14][15][16]. It also showed immunomodulatory effects [17] and alleviated serum glucose levels in patients suffering from diabetes [18], indicating health promotion or disease prevention properties. Recently, efforts have been intensified to conjugate polyphenols (PPNs) with CHOS to improve their physicochemical and biological properties. Furthermore, CHOS derivatives have been incorporated into food and food products for quality improvement and shelf-life extension.
2. Preparation of CHOS Derivatives
2.1. Carboxylated CHOS
Carboxylated CHOS (C-CHOS) was prepared by grafting the carboxyl group (COCH2CH2COO-) to the amino site at C-2 of the CHOS [52][19]. The CHOS (6.5 g) was solubilized in 10% acetic acid (50 mL) and mixed with fifteen milliliters of methanol. Later, to prepare C-CHOS with various levels of substitution, succinic anhydride (6.6 g) was dissolved in acetone and added to the aforementioned mixture for 1 h at room temperature. At a pH of 9.0–10.0, the reaction mixture was agitated for 4 h. The pH was maintained using sodium carbonate. The degree of substitution of the carboxyl group per CHOS monomer was 0.90 [52][19]. However, DS and site specificity including the -NH2 and -OH groups denoted as N and O at C2 and C6, respectively, are affected by reaction conditions [53][20]. Therefore, the laccase/2, 2, 6, 6-tetramethylpiperidine-1-oxyl (TEMPO) oxidation system was applied, and only the hydroxyl group at C-6 of the CHOS could be oxidized into carboxyl groups. 6-carboxylate chitooligosaccharide (6-CCHOS) was prepared using the aforementioned method, in which a carboxylate ion content of 2.3 mmol/g 6-CCHOS was obtained [54][21]. The synthesis of the C-CHOS was confirmed using NMR (1H-NMR and 13C-NMR) [52][19]. 1H-NMR showed protons associated with -CH3, C1–6, and -CH2CH2- at 1.9, 2.6, 3.3–3.6, 4.5, and 2.4 ppm, respectively. 13C-NMR showed carbon related to N-CH3, -CH2CH2- close to the carboxyl group, C-2, C-6, C-3, C-4, C-5, C-1, amide C=O, and C=O at 22, 32, 56, 61, 69, 73, 76, 78, 102, 174, 176, and 180 ppm, respectively. Moreover, FTIR spectra showed peaks associated with hydroxyl groups at 3408 cm−1; alkyl stretching at 2931 and 2860 cm−1; ester C=O at 1723 cm−1; amide C=O at 1653 cm−1; carboxyl C=O at 1565 and 1408 cm−1, and a pyranose ring at 1112, 1068, and 1030 cm−1. Additionally, the derivatization of the CHOS to 6-CCHOS was confirmed via FTIR, in which a higher absorption intensity at 3363 cm−1 was found compared to the native CHOS. The band was observed at 1643 cm−1. This was due to carboxylate (C=O) and the absence of the amide I band [54][21]. Moreover, the peak associated with -COO appeared at 170 ppm when the 13C-NMR spectra were analyzed. These modifications led to the formation of hydrogen bonds connecting with hydrophilic groups.2.2. Amino-Derived CHOS
Amino-derived CHOSs, namely aminoethyl CHOS (AE-CHOS), dimethylaminoethyl CHOS (DMAE-CHOS), or diethylaminoethyl CHOS (DEAE-CHOS), were prepared using 2-chlorethylamino hydrochloride, 2-dimethylamino-ethylchloride hydrochloride, or 2-diethylamino-ethylchloride hydrochloride, respectively [55,56,57,58][22][23][24][25]. The CHOS (0.40 g) was combined with the aforesaid amino solution (3.0 M; 20 mL) and mixed at 40 °C. After stirring the reaction mixture for 48 h, 3.0 M NaOH (20 mL) was added dropwise and filtered. The reaction mixture was then dialyzed against water after being acidified with 0.1 N of HCl. After the derivatization of the CHOS, the hydroxyl group at the C-6 position was replaced by the amino group due to its highest reactivity for aminoethylation [55][22]. In the FTIR spectrum of the synthesized CHOS amino derivatives, the peaks of absorptions at 2965 cm−1 and 1000–1150 cm−1 due to C–H stretching and C–O–C stretching were observed, respectively, indicating the substitution of the hydroxyl group at C-6 of the CHOS by AE, DMAE, and DEAE for AE-CHOS, DMAE-CHOS, and DEAE-CHOS, respectively. When an amino-derived CHOS was confirmed via 1H NMR, a peak appeared at 2.8 ppm, corresponding to the proton of –CH2N; a peak for protons of the acetyl group of the CHOS appeared at around 2 ppm; and peaks appeared at 2.9–3.6 ppm for protons of the pyranose unit superimposed with -NH2 of the aminoethyl group.2.3. Sulfated CHOS
Sulfated CHOS (S-CHOS) was prepared by adding sulfonated groups to C-3 and C-6 of CHOS [59][26]. First, solution I was prepared by mixing the CHOS (1.5 g), dimethylformamide (DMF; 60 mL), and dichloroacetic acid for 24 h. To make a sulfonated reagent (solution II), chlorosulfonic acid (10 mL) was gradually added to DMF (60 mL) at 0–4 °C. After reaching 60 °C, solution II was progressively combined with solution I. The reaction was run for 2 h. Subsequently, 100 mL of distilled water was added, and NaOH (20%, w/v) was used to change the pH to 7.0. After centrifugation, the supernatant was collected and precipitated with ethanol. After that, water was added to the precipitate, and it was dialyzed. S-CHOS powder was produced by lyophilizing the concentrated solution [59][26]. Wang et al. [60][27] also made S-CHOS. In brief, chlorosulfonic acid (3.4 mL) was poured dropwise into an ice-cold three-necked flask containing DMF (17 mL). To obtain a sulfation reagent, the reaction was performed at room temperature for 1.5 h. Then, 1 g of CHOS was added to the sulfation reagent and agitated for 1 h at 70 °C. Ethanol was used to precipitate the reaction and repeatedly rinsed. The precipitate was dissolved in distilled water and the pH was adjusted to 8.0 using NaHCO3 solution. The resultant solution was dialyzed and lyophilized to obtain S-CHOS [60][27]. NMR and FTIR were used to analyze the basic structure of the S-CHOS as well as to identify the sulfate groups in the S-CHOS. The FTIR spectra revealed bands characteristic of the S-CHOS at 1221 and 813 cm−1, which were attributable to the S=O and C-O-S bonds, respectively. The hydroxyl groups were therefore successfully sulfated. After sulfation, the signals of C-6 in the 13C NMR spectra of the S-CHOS were displaced to 66.8 ppm from 59.9 ppm in the CHOS. Furthermore, the signal at 60.0 ppm for unsubstituted C-6 demonstrated partial sulfation. The sulfate substitution in the S-CHOS was 1.02, implying that both C-2 and C-3 were also largely sulfated throughout the process.2.4. Quaternized CHOS
Quaternized CHOS (Q-CHOS) was synthesized via the conjugation of quaternary ammonium salt such as glycidyltrimethylammonium chloride (GTMAC) to the amino group at C-2 of CHOS under neutral and alkaline conditions. The hydroxyl group could react with the epoxide ring. Consequently, quaternization was carried out in an acidic environment. Feng et al. [61][28] prepared a Q-CHOS as follows: a CHOS (1 g) was dissolved in distilled water (20 mL) and mixed with three molar equivalents of GTMAC. Thereafter, the pH of the mixture was adjusted to 5.5 using acetic acid and heated at 50 °C for 12 h, and then the solution was precipitated using acetone followed by filtration and drying at 50 °C for 24 h. Also, Kim et al. [62][29] prepared a Q-CHOS using a similar method. The FTIR spectra of the Q-CHOS showed characteristic bands as follows [62][29]: The amino group’s interaction with the GTMAC epoxide had an absorption peak at 1516 cm−1, showing a lower intensity, and a new peak caused by the methyl groups of GTMAC was discovered at 1479 cm−1. All of the pyranose ring protons for the Q-CHOS’s 1H-NMR were found between 3.3 and 4.6 ppm. At 3.4 and 2.5 ppm, proton signals from two types of methylene groups were detected, respectively. Moreover, a peak at 4.3 ppm was attributed to methine protons. In addition, protons of the trimethylammonium group appeared at a peak of 3.2 ppm in the Q-CHOS spectra.2.5. N-Aryl CHOS
The synthesis of the N-aryl CHOS (aryl-CHOS) included a two-step reaction, in which the first step involved the formation of a Schiff base as an intermediate product and its reduction to aryl-CHOS occurred in the second step. CHOS (1%, w/v) was dissolved in ethanol (69%, v/v), followed by an adjustment of the pH to 5.0 using acetic acid (1%, v/v). The Schiff base was then created by adding 4-hydroxybenzaldehyde to the mixture and stirring for 12 h at room temperature. The reaction mixture was then added with 0.1 g of NaBH4 and agitated for 12 to 14 h. NaOH (15%, w/v) was used to neutralize the reaction mixture and centrifuged to separate the precipitate. Using dialysis membranes, the precipitate was dialyzed against distilled water. To eliminate the free aldehyde, the dialysate was precipitated in acetone and rinsed with diethyl ether. The final step was to dry the product at room temperature to produce 4-hydroxybenzyl-CHOS [63][30]. According to the FTIR spectra of 4-hydroxybenzyl-CHOS, characteristic bands appeared at 3195 cm−1 and were assigned to the C-H stretching of a benzene ring, whereas bands at 1506 and 1462 cm−1 indicated C-C bonds of a benzene ring. From the 1H-NMR spectra, 4-hydroxybenzyl-CHOS showed peaks at 8–6.9 ppm belonging to the protons of the aromatic ring of 4-hydroxybenzaldehyde along with characteristic peaks of CHOS. Thus, the spectroscopic results elucidated the successful link between 4-hydroxybenzaldehyde and CHOS [63][30].2.6. Polyphenol (PPN)- or Phenolic Acid (PA)-Conjugated CHOS
Currently, the interest in PPNs or PAs has been rising because of their ubiquitous nature and health benefits. Moreover, CHOS was grafted with PPNs or PAs due to their excellent bioactivities such as their antioxidant, antimicrobial, antidiabetic, and anti-cancer properties [64][31]. In addition, enhanced bioactivities of CHOS were reported after conjugation with PPNs or PAs. CHOS-PPN or CHOS-PA conjugates were synthesized through a carbodiimide-based chemical coupling method and free radical grafting reaction [6]. In the carbodiimide-based chemical coupling method, carbodiimides such as 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) or N,N′-dicyclohexylcarbodiimide (DCC) are used. EDC or DCC can activate the carboxyl group to form an O-acylisourea intermediate, followed by coupling with the primary amine of a CHOS to yield amide bonds. Vo et al. [65][32] prepared a CHOS-gallic acid conjugate. Firstly, CHOS (MW: 3–5 kDa; 2.50 g) was dissolved in distilled water (20 mL) and methanol (40 mL). Then, the pH of the mixture was adjusted to 6.8 to produce so-called solution A. Simultaneously, gallic acid (0.94 g) was dissolved in methanol (10 mL) and mixed with a DCC-methanol mixture (1.0315 g DCC in 10 mL methanol), known as solution B. Both solutions (A and B) were gradually mixed at 30 °C for 5 h. Thereafter, the reaction mixture was filtered to remove dicyclohexyl urea, which was formed as a side product, and the remaining solution was kept at 2 °C overnight. Subsequently, the remaining solution was precipitated using diethyl ether, and the precipitate was dissolved in distilled water (20 mL), followed by dialysis to remove the free gallic acid. CHOS-gallic acid conjugate powder with a yield of 43.5% was obtained after the lyophilization of the dialysate [65][32]. Nevertheless, O-acylisourea is the intermediate product, which is prone to hydrolysis, and it regenerates into a carboxyl group [66][33]. It could lower the grafting efficacy. To conquer this, other coupling reagents such as N-hydroxysuccinimide (NHS) and 1-hydroxybenzotriazole (HOBt) were introduced to enhance the grafting efficiency and avoid side reactions. NHS or HOBt can convert unstable O-acylisourea to ester, which finally reacts with the amino and hydroxyl groups of CHOS to yield a CHOS-PPN or CHOS-PA conjugate. Eight different PAs including caffeic, ferulic, 4-hydroxybenzoic, protocatechuic, p-coumaric, syringic, sinapinic, and vanillic acids were conjugated with CHOS [67][34]. A CHOS (1 g) was dissolved in 100 mL of methanol (20%, w/v). Subsequently, the aforementioned PAs were mixed with one equivalent of dicyclohexylcarbodiimide (DCC) and 1-hydroxybenzotriazole (HOBt) each and three equivalents of triethylamine (TEA). The mixture was transferred into a CHOS solution and stirred at room temperature for 24 h. The resultant solution was precipitated and washed using acetone, followed by lyophilization to obtain a PA-conjugated CHOS. The degree of substitution was in the range of 5.7–10.3%, depending on the PA used for conjugation. Moreover, the grafting of PAs on the CHOS was confirmed with the aid of 1H-NMR, in which peaks associated with the aromatic proton signals were observed between 6.0 and 8.0 ppm. In addition, PA-acylated CHOS derivatives were also produced using the carbodiimide coupling method [68][35]. The reaction was governed via amide bonding using the EDC/NHS catalytic system. PAs, including gallic acid, ferulic acid, p-coumaric acid, caffeic acid, protocatechuic acid, sinapic acid, and salicylic acid (25 mM each), were dissolved separately in deionized water or anhydrous ethanol at pH 5.0. Subsequently, 50 mM of EDC and 50 mM of NHS were added into the aforementioned solution and mixed for 2 h, followed by the addition of a CHOS (10 mM). After being stirred for 24 h at room temperature, precipitation was carried out using ethanol. The PA-acylated CHOS was obtained via the lyophilization of the precipitate [68][35]. The carbodiimide-based chemical coupling method generally requires a large amount of chemical crosslinkers in the reaction. Moreover, these crosslinkers are expensive and have hazardous impacts on the environment. They may cause adverse impacts on humans. Thus, their usage in the food and pharma sectors is limited [6]. The free-radical-induced grafting method was discovered as an alternative and successful conjugation, and it has been widely applied for the grafting of PPNs with CHOSs (Figure 1). The chemicals utilized in this process are affordable, less harmful, and environmentally benign, which can increase CHOS applications in the biomedical and food industries. Therefore, it could be an effective alternative for CHOS grafting with PPNs. Ascorbic acid is subjected to oxidation for ascorbate ion formation, which further donates two hydrogen ions or electrons in the presence of H2O2. Ascorbate is ultimately transformed into the resonance-stabilized tricarbonyl ascorbate free radicals via ascorbate free radicals [69][36]. With a pKa of -0.86, these ascorbate free radicals exist as tricarbonyl ascorbate free radicals because they cannot be protonated [70][37]. The previously generated H-ion or electron then interacted with H2O2 to make hydroxyl radicals. Thereafter, to create CHOS-macroradicals, the hydroxyl radical was used to extract hydrogen from the functional groups (amino and hydroxyl) of the CHOS [70][37]. In the end, the PPN molecules formed covalent bonds with the CHOS macro-radicals, resulting in the production of the CHOS-PPN conjugate.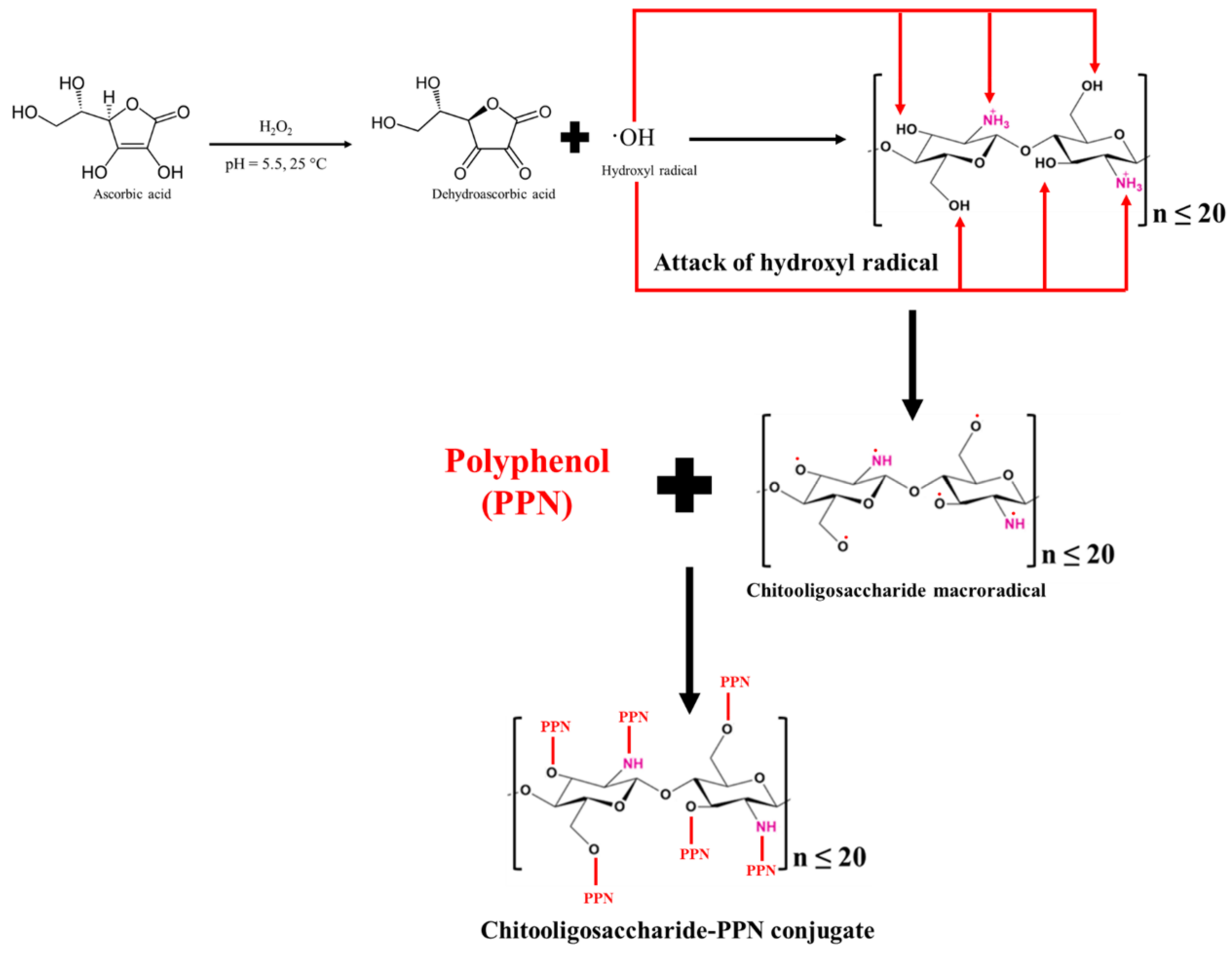
Figure 1. Flowchart for the formation of chitooligosaccharide-polyphenol conjugate using free radical grafting method.
2.7. Amphiphilic CHOS
In relation to their regulated rates of biodegradation and possible uses in drug administration, the production of amphiphilic biocompatible and biodegradable copolymers has attracted attention. Polycaprolactone (PCL) is a hydrophobic polymer that is extensively used as the implantable carrier for drug delivery systems. Thus, PCL was used to prepare an amphiphilic CHOS, which possessed self-assembly properties to encapsulate several drugs. Wang et al. [76][44] prepared a CHOS-PCL in two steps. Firstly, the functional groups (hydroxyl and amino groups) of the CHOS were selectively protected by hexamethyldisilazane (HMDS). Thereafter, a CHOS, either trisilylated or disilylated, at C-3 was dissolved in an organic solvent, such as chloroform or xylene or a mixture of both, with PCL and stannous octoate under N2. Then, the reaction mixture was kept at 120 °C for 24 h with continuous agitation. The resulting graft copolymer (CHOS-PCL) was then stirred at room temperature while being deprotected with the isopropyl alcohol/H2O/HCl solution. The maximum DS of 47.3% was determined using 1H NMR when PCL was grafted on the CHOS at different molar ratios. In addition, methylene proton signals of PCL were observed at 4.1, 2.3, 1.7, and 1.4 ppm along with proton signals associated with the methine and methylene of the CHOS at 3.0–5.0 ppm, thus confirming the grafting of PCL with the CHOS [76][44]. Some fatty acids such as arachidic acid (AA), stearic acid (SA), etc., were used for conjugation with CHOS to achieve amphiphilic properties. An amphiphilic CHOS-AA conjugate was synthesized [77][45]. Separately, at 50 °C for 15 min, CHOS (0.2 mM) and AA (0.6 mM) were dissolved in 20 mL of DMSO. Then, at room temperature, AA’s carboxyl groups were activated by adding EDC and NHS (1.5 mol/mol of AA). An activated AA solution was added dropwise to the CHOS solution within 5 min. Following a 12 h-incubation period for the coupling process, 4 mL of deionized distilled water was added to the reaction mixture. After applying 1 N of HCl to change the mixture’s pH to 3.5, it was agitated for an additional 30 min. Acetone was added to the mixture for precipitation. Free arachidic acid was then removed via centrifugation. The precipitate was dispersed with distilled water and dialyzed using a dialysis membrane against distilled water for 24 h. Finally, the dialyzed products were lyophilized [77][45]. The amine peak at 1591 cm−1 of the CHOS was replaced by a new absorption band at 1558 cm−1, which was connected to the development of a new amide bond (amide II band) based on the FTIR spectrum of the CHOS-AA conjugate. Additionally, the CHOS-AA conjugate’s absorption bands at 2918 and 2851 cm−1 indicated the stretching vibrations of the acyl chain and CH2 and CH3 of AA. Additionally, 1H-NMR indicated proton signals for both the CHOS and AA, suggesting the conjugation of AA to the CHOS. The conjugation was confirmed with the peak of CH2 associated with a carbonyl group of AA at 2.07 ppm, whereas that peak disappeared in the spectrum of a physical mixture of CHOS and AA [77][45]. In a similar manner, SA was grafted with a CHOS when EDC was present [78][46]. While stearic acid (0.5 g) was dissolved in 20 mL of ethanol, CHOS (0.4 g) was solubilized in 30 mL of distilled water. At room temperature, 2 g of EDC was added to the CHOS solution, which was then heated to 90 °C while being vigorously stirred. Parallelly, the SA solution was added dropwise. The final reaction mixture was stirred at 90 °C for 5 h, cooled to room temperature, and further stirred for 24 h. After the reaction was finished, the mixture was dried at 50 °C in a vacuum oven, and the residue was dissolved in 20 mL of ethanol. To eliminate the unreacted SA, the precipitate was filtered and then collected [78][46]. Finally, the precipitate was dissolved in distilled water, followed by dialysis and lyophilization, respectively. The peaks of amide I and II at 1640 and 1560 cm−1, respectively, were due to the amide band between the CHOS and SA, along with the absence of an absorption peak associated with the carboxyl groups of SA at 1700 cm−1 in the FTIR spectra of the CHOS-SA conjugate. This confirmed the successful grafting. Moreover, proton signals at 0.9 and 1.0 ppm due to the methyl and methylene hydrogen of the stearate group in the CHOS-SA also verified the grafting of SA on the CHOS [79][47]. In addition, the degree of substitution was 15.4%, as determined using the 2,4,6-trinitrobenzene sulfonic acid method. Hu et al. [80][48] further conjugated CHOS-SA with the antitumor drug, i.e., doxorubicin (DOX). The conjugation was confirmed via 1H-NMR, in which the proton of the anthracene of DOX at 8.0 ppm in DOX-CHOS-SA was recorded.2.8. Other CHOS Derivatives
Guanidinylated CHOS was prepared by dissolving CHOS hydrochloride (1 g), 1-amidinopyrazole hydrochloride (2.70 g; 18.4 mmol), and trimethylamine (3.34 g; 33.0 mmol) in water for 7 days [81][49]. The mixture was precipitated using isopropanol and collected via filtration, followed by vacuum drying. Guanidinylated CHOS showed characteristic absorption peaks for the C=N stretching vibration and NH bending vibration of guanidino groups at approximately 1725 cm−1 and 1590 cm−1, respectively. Moreover, the 13C-NMR spectrum showed signals corresponding to quaternary carbons in the guanidino groups at 159.3 ppm [81][49].References
- Yan, N.; Chen, X.J.N. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157.
- Kandra, P.; Challa, M.M.; Jyothi, H.K.P. Efficient use of shrimp waste: Present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29.
- Klomklao, S.; Poonsin, T.; Benjakul, S.; Simpson, B.K. Byproducts from Shellfish Harvesting and Processing. In Byproducts from Agriculture and Fisheries; Simpson, B.K., Aryee, A.N.A., Toldrá, F., Eds.; Wiley: Hoboken, NJ, USA, 2019.
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56.
- Rakkhumkaew, N.; Pengsuk, C. Chitosan and chitooligosaccharides from shrimp shell waste: Characterization, antimicrobial and shelf life extension in bread. Food Sci. Biotechnol. 2018, 27, 1201–1208.
- Singh, A.; Mittal, A.; Benjakul, S. Chitosan, chitooligosaccharides and their polyphenol conjugates: Preparation, bioactivities, functionalities and applications in food systems. Food Rev. Int. 2023, 39, 2297–2319.
- Liang, S.; Sun, Y.; Dai, X. A Review of the preparation, analysis and biological functions of chitooligosaccharide. Int. J. Mol. Sci. 2018, 19, 2197.
- Guan, Z.; Feng, Q. Chitosan and chitooligosaccharide: The promising non-plant-derived prebiotics with multiple biological activities. Int. J. Mol. Sci. 2022, 23, 6761.
- Singh, A.; Benjakul, S.; Prodpran, T. Chitooligosaccharides from squid pen prepared using different enzymes: Characteristics and the effect on quality of surimi gel during refrigerated storage. Food Prod. Process. Nutr. 2019, 1, 5.
- Tabassum, N.; Ahmed, S.; Azam Ali, M. Chitooligosaccharides for Drug Delivery. In Chitooligosaccharides; Kim, S.K., Ed.; Springer: Cham, Switzerland, 2022.
- Sinha, S.; Tripathi, P. Disease Preventing Bioactivities of Chitooligosaccharides: Current Status and Future Trends. In Chitooligosaccharides; Kim, S.K., Ed.; Springer: Cham, Switzerland, 2022.
- Shen, K.-T.; Chen, M.-H.; Chan, H.-Y.; Jeng, J.-H.; Wang, Y.-J. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem. Toxicol. 2009, 47, 1864–1871.
- Anil, S. Potential medical applications of Chitooligosaccharides. Polymers 2022, 14, 3558.
- Rajabi, M.; Cabral, J.D.; Saunderson, S.; Ali, M.A. 3D printing of chitooligosaccharide-polyethylene glycol diacrylate hydrogel inks for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2023, 111, 1468–1481.
- Ratanavaraporn, J.; Kanokpanont, S.; Tabata, Y.; Damrongsakkul, S. Growth and osteogenic differentiation of adipose-derived and bone marrow-derived stem cells on chitosan and chitooligosaccharide films. Carbohydr. Polym. 2009, 78, 873–878.
- He, B.; Wang, J. Chitooligosaccharides prevent osteopenia by promoting bone formation and suppressing bone resorption in ovariectomised rats: Possible involvement of COX-2. Nat. Prod. Res. 2015, 29, 359–362.
- Long, T.; Yu, J.; Wang, J.; Liu, J.; He, B.-S. Orally administered chitooligosaccharides modulate colon microbiota in normal and colitis mice. Int. J. Pharmacol. 2018, 14, 291–300.
- Liu, B.; Liu, W.-S.; Han, B.-Q.; Sun, Y.-Y. Antidiabetic effects of chitooligosaccharides on pancreatic islet cells in streptozotocin-induced diabetic rats. World J. Gastroenterol. WJG 2007, 13, 725.
- Rajapakse, N.; Kim, M.-M.; Mendis, E.; Kim, S.-K. Inhibition of free radical-mediated oxidation of cellular biomolecules by carboxylated chitooligosaccharides. Bioorg. Med. Chem. 2007, 15, 997–1003.
- Bukzem, A.L.; Signini, R.; Dos Santos, D.M.; Lião, L.M.; Ascheri, D.P.R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624.
- Bu, X.; Pei, J.; Zhang, F.; Liu, H.; Zhou, Z.; Zhen, X.; Wang, J.; Zhang, X.; Chan, H. The hydration mechanism and hydrogen bonding structure of 6-carboxylate chitooligosaccharides superabsorbent material prepared by laccase/TEMPO oxidation system. Carbohydr. Polym. 2018, 188, 151–158.
- Ngo, D.-N.; Qian, Z.-J.; Je, J.-Y.; Kim, M.-M.; Kim, S.-K. Aminoethyl chitooligosaccharides inhibit the activity of angiotensin converting enzyme. Process Biochem. 2008, 43, 119–123.
- Ngo, D.H.; Ngo, D.N.; Kim, S.-K.; Vo, T.S. Antiproliferative effect of aminoethyl-chitooligosaccharide on human lung A549 cancer cells. Biomolecules 2019, 9, 195.
- Karagozlu, M.Z.; Karadeniz, F.; Kong, C.-S.; Kim, S.-K. Aminoethylated chitooligomers and their apoptotic activity on AGS human cancer cells. Carbohydr. Polym. 2012, 87, 1383–1389.
- Yoon, N.Y.; Ngo, D.-N.; Kim, S.-K. Acetylcholinesterase inhibitory activity of novel chitooligosaccharide derivatives. Carbohydr. Polym. 2009, 78, 869–872.
- Liu, Y.; Yu, F.; Zhang, B.; Zhou, M.; Bei, Y.; Zhang, Y.; Tang, J.; Yang, Y.; Huang, Y.; Xiang, Q. Improving the protective effects of aFGF for peripheral nerve injury repair using sulfated chitooligosaccharides. Asian J. Pharm. Sci. 2019, 14, 511–520.
- Wang, S.; Luo, Y.; Huang, L.; Wang, S.; Hao, C.; Sun, L.; Zhang, Y.; Wang, W.; Li, C. The inhibition effects and mechanisms of sulfated chitooligosaccharides on influenza A virus in vitro and in vivo. Carbohydr. Polym. 2022, 286, 119316.
- Feng, H.; Xia, W.; Shan, C.; Zhou, T.; Cai, W.; Zhang, W. Quaternized chitosan oligomers as novel elicitors inducing protection against B. cinerea in Arabidopsis. Int. J. Biol. Macromol. 2015, 72, 364–369.
- Kim, J.Y.; Lee, J.K.; Lee, T.S.; Park, W.H. Synthesis of chitooligosaccharide derivative with quaternary ammonium group and its antimicrobial activity against Streptococcus mutans. Int. J. Biol. Macromol. 2003, 32, 23–27.
- Trinh, M.D.L.; Dinh, M.-H.; Ngo, D.-H.; Tran, D.-K.; Tran, Q.-T.; Vo, T.-S.; Ngo, D.-N. Protection of 4-hydroxybenzyl-chitooligomers against inflammatory responses in Chang liver cells. Int. J. Biol. Macromol. 2014, 66, 1–6.
- Mittal, A.; Singh, A.; Zhang, B.; Visessanguan, W.; Benjakul, S. Chitooligosaccharide conjugates prepared using several phenolic compounds via ascorbic acid/H2O2 free radical grafting: Characteristics, antioxidant, antidiabetic, and antimicrobial activities. Foods 2022, 11, 920.
- Vo, T.-S.; Ngo, D.-H.; Bach, L.G.; Ngo, D.-N.; Kim, S.-K. The free radical scavenging and anti-inflammatory activities of gallate-chitooligosaccharides in human lung epithelial A549 cells. Process Biochem. 2017, 54, 188–194.
- Madison, S.A.; Carnali, J.O. pH optimization of amidation via carbodiimides. Ind. Eng. Chem. Res. 2013, 52, 13547–13555.
- Eom, T.-K.; Senevirathne, M.; Kim, S.-K. Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ. Toxicol. Pharmacol. 2012, 34, 519–527.
- Sun, Y.; Ji, X.; Cui, J.; Mi, Y.; Zhang, J.; Guo, Z. Synthesis, characterization, and the antioxidant activity of phenolic acid chitooligosaccharide derivatives. Mar. Drugs 2022, 20, 489.
- Singh, A.; Benjakul, S.; Huda, N.; Xu, C.; Wu, P. Preparation and characterization of squid pen chitooligosaccharide–epigallocatechin gallate conjugates and their antioxidant and antimicrobial activities. RSC Adv. 2020, 10, 33196–33204.
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938.
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639.
- Van Bui, H.; Ngo, D.-N. The research determines appropriate parameters in the synthesis process of syringic acid grafted chitooligosaccharides. VNUHCM J. Sci. Technol. Dev. 2019, 22, 317–323.
- Bui, V.-H.; Vo, H.-T.N.; Ngo, D.-N. Antioxidant effect of syringic acid grafted chitooligosaccharides in RAW264. 7 Cells. In Vietnam: Healthcare Technology for Smart City in Low-and Middle-Income Countries, Proceedings of the 8th International Conference on the Development of Biomedical Engineering in Vietnam: Proceedings of BME 8, Online, 20–22 July 2020; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 501–516.
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Gallic acid-grafted chitooligosaccharides suppress antigen-induced allergic reactions in RBL-2H3 mast cells. Eur. J. Pharm. Sci. 2012, 47, 527–533.
- Yuan, Y.; Tan, W.; Lin, C.; Zhang, J.; Li, Q.; Guo, Z. Development of antioxidant chitosan-based films incorporated with chitooligosaccharide-caffeic acid conjugates. Food Hydrocoll. 2023, 138, 108431.
- Benjakul, S.; Singh, A.; Mittal, A. Chitooligosaccharides: Preparation and applications in food and nutraceuticals. In Chitooligosaccharides: Prevention and Control of Diseases; Kim, S., Ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2022; pp. 203–221.
- Wang, C.; Li, G.; Tao, S.; Guo, R.; Yan, Z. Crystalline and micellar properties of amphiphilic biodegradable chitooligosaccharide-graft-poly (ε-caprolactone) copolymers. Carbohydr. Polym. 2006, 64, 466–472.
- Termsarasab, U.; Cho, H.-J.; Kim, D.H.; Chong, S.; Chung, S.-J.; Shim, C.-K.; Moon, H.T.; Kim, D.-D. Chitosan oligosaccharide–arachidic acid-based nanoparticles for anti-cancer drug delivery. Int. J. Pharm. 2013, 441, 373–380.
- Hu, F.-Q.; Zhao, M.-D.; Yuan, H.; You, J.; Du, Y.-Z.; Zeng, S. A novel chitosan oligosaccharide–stearic acid micelles for gene delivery: Properties and in vitro transfection studies. Int. J. Pharm. 2006, 315, 158–166.
- Hu, F.-Q.; Ren, G.-F.; Yuan, H.; Du, Y.-Z.; Zeng, S. Shell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxel. Colloids Surf. B Biointerfaces 2006, 50, 97–103.
- Hu, F.-Q.; Liu, L.-N.; Du, Y.-Z.; Yuan, H. Synthesis and antitumor activity of doxorubicin conjugated stearic acid-g-chitosan oligosaccharide polymeric micelles. Biomaterials 2009, 30, 6955–6963.
- Izawa, H.; Kinai, M.; Ifuku, S.; Morimoto, M.; Saimoto, H. Guanidinylation of chitooligosaccharides involving internal cyclization of the guanidino group on the reducing end and effect of guanidinylation on protein binding ability. Biomolecules 2019, 9, 259.
More
