Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 3 by Lindsay Dong.
The treatment of petroleum refinery wastewater (PRWW) is of great interest in industrial wastewater management. This wastewater contains a diverse concentration of contaminants such as oil and grease, petroleum hydrocarbons, phenols, ammonia, and sulfides, as well as other organic and inorganic composites. Refinery wastewater treatment has been attempted through various processes, including physical, biological, chemical, and hybrid methods, which combine two or more techniques.
- wastewater
- petroleum refinery
- treatment process
- physiochemical
1. Introduction
Water is one of the most valuable natural resources in the world and alongside air and soil, supports our environmental ecosystem. It is a vital resource for a variety of human activities from domestic to industrial applications and provides the living environment for marine biodiversity. Despite this integral support, global industrialization and technological advancement are associated with generating different types of wastewater released into our environmental ecosystems [1]. Besides that, the demand for petroleum resources has continued to rise in many parts of the world to enhance the economy. However, the petroleum production and refining process is also associated with the generation of large volumes of wastewater which are highly toxic even at low concentrations [2][3]. The impact of environmental pollution is widely manifested in different areas including affecting aquatic life, destroying natural land for agricultural production, and contaminating groundwater resources [4]. On the other hand, the treatment of this wastewater is also becoming a growing challenge in the petroleum industry due to its complex and dynamic nature. Hence, different treatment techniques including physical, chemical, biological, or hybrid processes have been employed and reported in the literature. However, many of these processes have their distinct advantages and disadvantages in terms of efficiency, energy requirements and treatment costs. Considering this, more research is necessary to explore the most appropriate treatment techniques that are cost-effective and environmentally friendly.
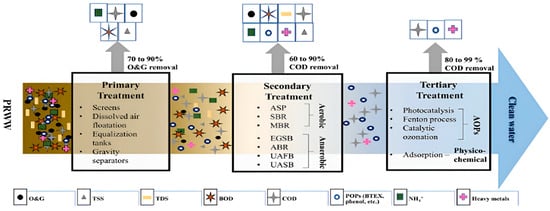
2. Characterization of Petroleum Refinery Wastewater
The composition of a typical petroleum refinery wastewater (PRWW) usually depends on the crude oil qualities as well as the complexity and process configuration of the petroleum refining industry. Petroleum crude oil is a complex mixture of hydrocarbon compounds of different carbon chains and other toxic organics such as phenols, which usually constitute the major organic pollutants in PRWW [3]. Generally, the PRWW contaminants can be classified into (a) oil and grease, (b) organic pollutants (which includes the hydrocarbons, organic compounds and all other biological oxygen demand (BOD) contaminants), (c) inorganic pollutants (including ammonia, nitrogen, phosphorus, chlorides and other inorganic salts), and (d) heavy metals [5]. According to Diya’uddeen et al. [6] and Whale et al. [7], the most significant contaminants that are of environmental concern include oil, phenols, suspended solids, metals, ammonia, dissolved minerals, and substances which are responsible for oxygen level depletion as a measure of (BOD) and chemical oxygen demand (COD). Therefore, PRWW treatment facilities are usually designed to be capable of the removal of both organic and inorganic contaminants. A typical PRWW effluent is usually characterized by high BOD and COD because of the overall distribution of the aliphatic and aromatic hydrocarbons, grease and emulsified oils, ammonia, cyanides and other inorganic substances from the crude oil composition. It is usually rich in hydrocarbons from the three main classes which include: (a) the paraffin comprising low-chain carbon atoms such as methane (CH4), ethane (C2H6) and propane (C3H8); (b) the naphthenes such as dimethyl cyclopentane and cyclohexane; and (c) the aromatic compounds comprising of the benzene compounds and its derivatives [8]. The aromatics are unsaturated hydrocarbons containing at least one or more benzene rings and their derivatives, generally referred to as BTEX (Benzene, Toluene, Ethyl Benzene and Xylene) which are mostly toxic to both human and aquatic species [8][9]. In most typical PRWW samples, the average reported values for BOD and COD can be up to 400 mg/L and 600 mg/L, respectively [3][10][11]. Rahi et al. [12] also reported about 150–250 mg/L, 300–600 mg/L, and 20–200 mg/L for BOD, COD and oil concentrations in desalted PRWW effluent, respectively. They further revealed that the oil concentration can reach up to 5000 mg/L in the effluents from the bottom of tanks with a concentration of about 1–100 mg/L of benzene. Due to their high environmental impact, discharge limits for the concentrations of various parameters are always established in every petroleum refining industry for policy compliance [13]. Different review and research articles on PRWW including [6][14][15][16][17] have reported the maximum concentration level (MCL) of the PRWW contaminants. Based on the reviews, the average effluent qualities reported for pH, BOD, COD, oil/grease, phenols, and total organic carbon (TOC) are 6–9, <20 mg/L, <200 mg/L, <10 mg/L, <0.25 mg/L, and <75 mg/L, respectively.3. Treatment of Petroleum Refinery Wastewater
Since petroleum wastewater contains toxic contaminants, which are a major threat to the environmental ecosystem, it is necessary to use the appropriate treatment before disposal and to meet the regulatory requirements. Given this, there are various treatment techniques which have been employed and reported in the literature for the treatment of PRWW [18]. While some already established technologies are efficient in terms of their treatment, cost and energy requirement, others are associated with high energy and maintenance costs and hence are not environmentally friendly. Therefore, efficiency assessment in terms of energy requirements, flexibility to treat various contaminants, and level of waste generation as a by-product at the end of the treatment process is critical to the development and application of any treatment technology [19]. Generally, PRWW treatment has two main stages: the pre-treatment stage, which is used to reduce contaminant loads such as oil, grease, and suspended solids. Secondly, there is degradation of the pollutants to an acceptable discharge limit [3][5][6]. Some reported treatment techniques in the literature include biological processes [20][21][22][23][24], coagulation processes [25][26][27], adsorption processes [20], membrane processes [28][29][30][31], chemical oxidation [32], and advanced oxidation processes (AOPS) [33][34][35][36]. In most cases, the determination of the treatment efficiency of these techniques focuses on the efficiency of the removal of the BOD, COD, oils and grease, phenols, sulfates, total organic carbon (TOC) as well as the concentration of heavy metals. Based on this, advanced oxidation processes such as Fenton oxidation and photocatalysis are receiving more attention nowadays due to their high capability to delete recalcitrant petroleum contaminants [5]. Many advances in treatment technologies have been achieved in recent years due to advancements in material science caused by a dynamic approach to the treatment of emerging contaminants [37].3.1. Conventional Treatment Techniques
PRWW effluent can be treated using either conventional, advanced, or integrated treatment processes (Figure 16). Conventional techniques have been widely used since the beginning of the 20th century and mainly consist of a combination of physical, chemical, and biological processes. According to Yu et al. [4], conventional techniques for the treatment of PRWW include flotation, coagulation, biological treatment, and membrane separation technology. However, these techniques are usually associated with various limitations including low efficiency, high capital operating cost as well as low sensitivity to emerging complex organic contaminants [38]. Toxic recalcitrant pollutants from hydrocarbon sources such as naphthenic acids (NAs) usually remain a considerable challenge in the treatment of PRWW using biological processes. Furthermore, due to their low efficiency and operational limitations, it makes it necessary to adopt more robust advanced treatment systems to achieve MCL. It usually includes a sequence of mechanical and physicochemical processes, followed by biological treatment of usually activated sludge treatment units. Some of the notable biological treatment systems in this regard, as indicated in Figure 1, include up-flow anaerobic sludge blankets (UASB), sequence batch reactors (SBR), membrane bioreactors (MBR), up-flow anaerobic fixed beds (UAFB), granular sludge beds (EGSB) and anaerobic baffled reactors (ABR).
Figure 1. General overview of the PRWW treatment techniques [39].
The pre-treatment stage is sometimes regarded as part of the primary treatment, where the majority of the suspended solids are separated and removed with the help of gravity, sedimentation, and filtration processes [15]. The advanced treatment systems include advanced oxidation processes, (such as photocatalysis, Fenton-oxidation, and electrochemical processes) and have been reported to provide more efficient treatment and less production of by-products that may also require further treatment. Alternatively, the use of an integrated or hybrid system which combines two or more advanced processes is also, nowadays, receiving more attention to provide the most effective treatment for the removal of oil and other hazardous pollutants from petroleum wastewater [40].
3.1.1. Physicochemical Processes
The physicochemical processes are a set of techniques that employ the use of the physical and chemical properties of the PRWW in the removal of contaminants. The conventional physical techniques usually include the use of filtration, floatation, adsorption, and sedimentation, while the chemical techniques include precipitation and coagulation processes [5]. The physical PRWW treatment process techniques such as screening, floatation, sedimentation, and gravity separation do not require the application of biological or chemical agents during the treatment process. They usually constitute the primary treatment process to reduce the waste load before proceeding to the secondary treatment units [5].
Flotation and Sedimentation Processes
The sedimentation process is used for the separation of water and oil due to the density difference. Hence, a significant density difference is required to provide an optimum separation. Oil and water sedimentation can be mechanically achieved using separators such as the API separator which operates on the principle of specific gravity differences to allow the settlement of heavy oil and pollutants [38]. Diffused air flotation (DAF) is achieved by introducing fine air bubbles to enhance the formation of a scum layer between the oil and the water for easy separation. The technique is achieved by introducing air under pressure which results in the pollutants rising to the top surface (Figure 2). High levels of total suspended solids, colloids, as well as some immiscible liquids are significantly reduced during this stage [41]. Abuhasel et al. [36] reported that DAF techniques enhanced by nanobubble systems were applied along with surfactants to reduce the surface tension of the oil concentration. About 90% oil separation efficiency was reported using this system. The technique has shown better efficiency than the traditional DAF system. Floatation and gravity separation were usually used as the first stage separation process to remove floating and dispersed oil efficiently. However, they are not efficient in terms of the separation of emulsified oil [37][42].
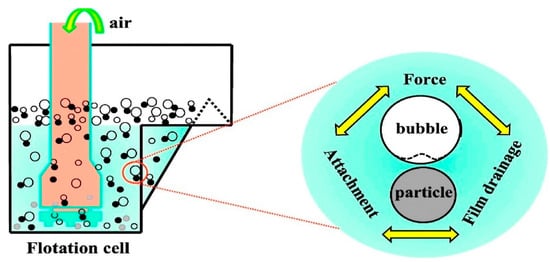

Figure 2. The mechanism of dissolved air flotation [37].
Coagulation/Flocculation
The use of coagulants to form flocs is also another physicochemical treatment process for the removal of pollutants from PRWW. Iwuozor [43] reported that coagulants are generally polyelectrolytes or synthetic organic polymers with high molecular weight which form multi-charged poly nuclear complexes in solution that makes flocs settle easily (Figure 3). The function of the coagulant is to promote the agglomeration or accumulation of wastewater particles by reducing the surface charges of the electrostatic particles. Different investigations have indicated the ability of the coagulation process for the treatment of PRWW. Coagulation processes were reported to be effective in treating heavy metals and high-level concentrations of organic pollutants.
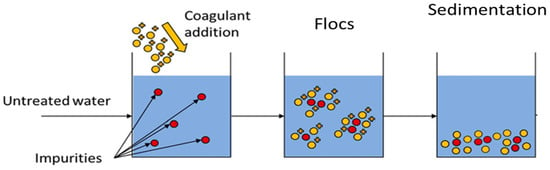

Figure 3. Illustration of the mechanisms of coagulation, flocculation and sedimentation [44].
Adsorption Using Conventional Adsorbents
The adsorption process can be both a conventional and advanced treatment technique depending upon the adsorbent material as indicated in Figure 4. Conventional adsorbent materials such as activated carbon, zeolites and silica have been used for a long time in the treatment of PRWW. Nowadays, adsorption techniques (using both conventional and non-conventional adsorbents) are one of the commonly studied techniques for industrial wastewater treatment due to their simplicity and lower treatment cost [45]. Additionally, besides its effectiveness and economic advantage, the adsorption technique is sometimes a reversible process where adsorbents can be regenerated simply through an appropriate desorption process [46]. Parameters affecting adsorption process efficiency include pH, temperature, contact time and adsorbent porosity and dose. The pH factor affects the ability of the hydrogen ions in the solution, their interaction with the functional groups and the metal ions in the case of heavy metals [47].
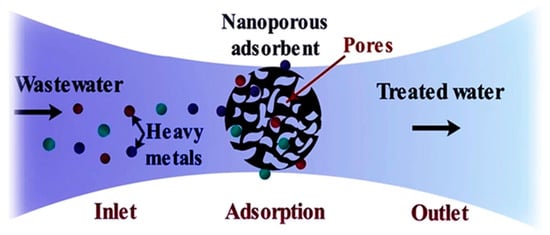

Figure 4. The process of adsorption using a nano-porous adsorbent for heavy metals [48].
Membrane Processes
The application of membrane technology for the treatment of wastewater has been in existence since the 18th century [49]. It is a physicochemical treatment technique that is gaining more acceptance nowadays and is also efficient in the treatment of organic matter. Membranes are used as a selective barrier (Figure 5) for the separation of two phases through a semi-permeable pore space by the restriction of movement between components [50]. The membrane mechanism in Figure 5 has shown the successful separation of the coloured contaminations represented in brown over the membrane surface. According to Ezugbe [50] and Aljuboury et al. [5], membranes can be generally classified into two main types; organic membranes (usually made from organic polymers) and inorganic membranes (made from silica, metals, zeolites, or ceramics). Depending on their pore sizes, membranes can be used for microfiltration ultrafiltration or nanofiltration [51].
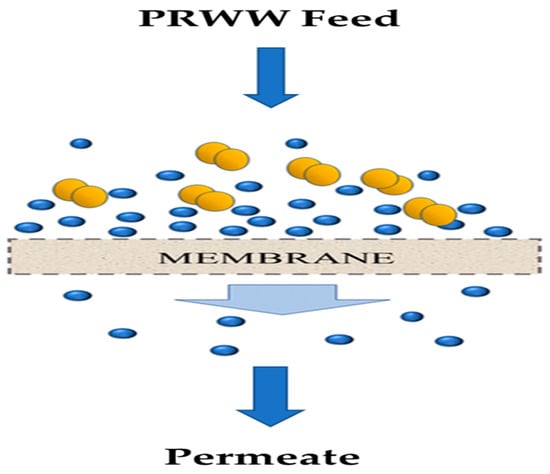

Figure 5. Illustration of the mechanism of a membrane process [52].
3.2. Chemical Processes
Chemical Precipitation and Ion Exchange
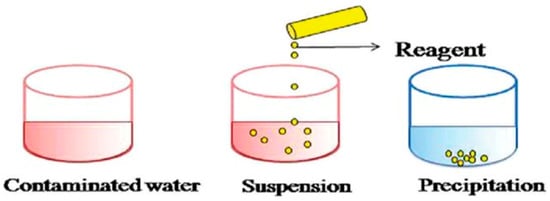
Chemical processes utilize the application of chemical reactions in the removal of contaminants from wastewater. Neutralization, ozonation, ion exchange and oxidation processes are among the most widely used chemical processes in the treatment of PRWW [53]. Neutralization consists of the use of an acid or base such as lime to adjust the pH level [5]. Generally, chemical precipitation is one of the most widely used conventional chemical processes for the removal of heavy metal concentrations from inorganic effluents [54]. In the precipitation process (Figure 6), heavy metal ions react with suitable chemicals called precipitants to form insoluble precipitates. which can be further separated by a sedimentation or filtration process [55]. It is a relatively simple and less costly technique which can be used for the removal of metals and sulfides. The coagulation precipitation method is broadly used with the help of chemical precipitants such as Ca(OH)2 and NaOH as indicated in equation 1 [48]. Alnakeeb and Rasheed [56] have reported the application of BaCl2 and Al (OH)3 in the treatment of PRWW from Al-Doura Refinery in Iraq. High sulfate removal efficiency was obtained with BaCl2 over Al (OH)3 and it was concluded that aluminium hydroxide is unsuitable for PRWW with neutral pH and low sulfate concentrations. Barium salts are highly insoluble making them an excellent precipitant for sulfate ions. Altaş and Büyükgüngör [57], also reported the use of Ca(OH)2 as a precipitant modified with Fe2+ ions and obtained 96–99% and 50–80% removal efficiencies for sulfide and COD, respectively.

Figure 6. Illustration of a chemical precipitation process of lead metal ions [58].
3.3. Biological Processes
Biological processes utilize the use of microbial activity from living organisms such as bacteria to decompose or degrade organic contaminants. The four major groups of biological treatment processes are aerobic, anaerobic, anoxic (the process by which nitrate is biologically converted into nitrogen gas in the absence of oxygen), or a combination of the three. The principal applications for these processes are removing carbonaceous organic matter (measured in BOD, COD, or TOC), nitrification, denitrification, or stabilization [59]. There are various biological processes which have been reported to be effective in treating PRWW, among which the activated sludge process is the most widely used [60]. However, there is little removal efficiency of petroleum hydrocarbons and a large amount of sludge production, but at least up to 60–90% COD removal efficiency was observed in many biological treatments of PRWW [39].
Furthermore, anaerobic digestion produces methane gas as a renewable energy and requires less space and sludge than the aerobic process. Before the application of the biological treatment techniques, pretreatment processes such as flotation, flocculation, sedimentation, and filtration are usually employed to eliminate the free oil and gross solids as well as increase biodegradability [61]. Different reactor systems for the aerobic process have been designed to treat PRWW including the traditional activated sludge system, contact stabilization active sludge, membrane bioreactor (MB), biological aerated filter (BAF), moving bed biofilm reactor (MBBR), sequence batch reactor (SBR) etc. Bioreactors adopted for PRWW are generally categorized as suspended growth, attached growth or hybrid process treatments [62].
3.3.1. Bioremediation Using Constructed Wetlands
The PRWW pollutants can also be combated using plant accumulation capabilities in the form of constructed wetlands. Phytoremediation is where plants alone and their associated microorganisms are used to degrade pollutants from contaminated systems. Based on this, constructed wetlands are widely used as a major technology in the restoration of oil-polluted environments to restore natural habitats [62]. Unlike physical and chemical processes, bioremediation is seen as a more environmentally friendly system due to its generation of less hazardous reaction products.
3.4. Advanced Treatment Processes
3.4.1. Adsorption Using Modified Adsorbents
With the advancements in the field of material science and the need for an effective and low-cost adsorbent, different natural and synthetic materials have been tested for the adsorption of contaminants from wastewater of different industrial effluents. Although the selection of an appropriate adsorbent material with suitable properties is indispensable in obtaining maximum adsorption capacity, the adsorption technique is often seen as the best choice in the treatment of different types of wastewater. Vikrant and Kim [63] maintained that this is because the adsorption technique is regarded as the simplest and most fitting treatment technique for almost all types of wastewater [64]. Additionally, the adsorption technique is sometimes also believed to be the optimal method even for crude oil spill clean-up because of its relatively low cost and high efficiency. Various oil hydrophobic adsorbents exist nowadays, such as natural sorbents, synthetic organic polymers, and inorganic mineral materials generated from a variety of sources that can be used to treat oily PRWW [64].
3.4.2. Electrochemical Techniques
Electrochemical technology is another promising treatment technology for the removal of organic pollutants from industrial wastewater using the application of electric currents supplied to electrodes. The major electrochemical techniques can occur in the forms of electrocoagulation, electro-floatation electro-oxidation, electro-Fenton electrodialysis, electrodeposition, and electrode ionization etc. [65][66]. Meanwhile, this technique also has its major challenges and limitations which affect its friendly application and efficiency. Adetunji and Olaniran [40] reported that electrochemical technologies are usually affected by operating conditions such as current density, pH, electrode materials, temperature, concentration and structure of phenols. However, no chemical addition is needed and there is less waste generation, the electrochemical process is considered a green technology which is simple to operate and integrate with other techniques [66]. The average estimated time for an electrochemical treatment process to treat PRWW is about 5–6 h. However, most of the studies conducted on electrochemical treatments were lab-scale processes with only a few evaluated at a pilot scale. Hence, there is a need for further efforts to determine the applicability of the prototype technology of the system to establish its viability [67].Electro Floatation (EF)
This is an advanced and enhanced form of air floatation process which carries floating contaminants to the surface by buoyancy and gas bubbles (usually oxygen and hydrogen gases) produced because of the electrolysis of water [66]. Unlike the conventional DAF which depends on the solubility of oxygen and nitrogen in the wastewater, in electro floatation oxygen and hydrogen gas bubbles are formed at the surface of the anode and cathode, respectively. Although it can be used as a separate process, it is usually combined with coagulation, flocculation, or both to remove contaminants by skimming [68]. The efficiency of the EF process is dependent on the current density, pH of solution and temperature. Furthermore, it differs from conventional air floatation in that it provides uniform and finely dispersed gas bubbles and requires little space and operational cost [40]. While the choice of the electrode material is vital to a successful implementation of EF, titanium-based inert anodes in the form of dimensional stable anodes (DAS) are the most dominantly used anodes [69].Electrocoagulation (EC)
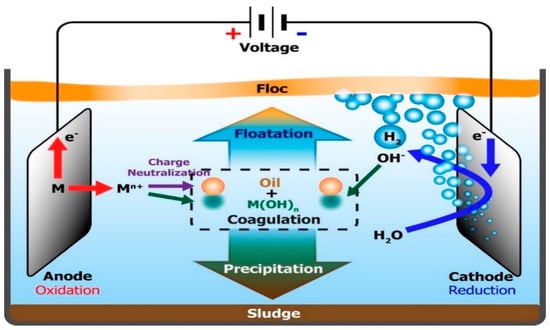
Electrocoagulation is one of the most prominent electrochemical processes employed in the removal of colloidal immiscible forms of pollutants of less than 10 μm [70]. This technology as indicated in Figure 7 involves an in-situ release of appropriate coagulant (such as aluminium or iron species) from a metal electrode with the application of an electric current. This process would lead to the electrolytic dissolution of the metal ions and result in a simultaneous formation of hydroxyl ions and hydrogen gas, while the coagulant aggregates and precipitates suspended solids [40]. Among the advantages of this technology are simple and automated operation, lower sludge volume and no chemical requirement, except for pH control [71]. Similarly, Akkaya [72] reported the use of aluminium and iron cathode electrodes obtained from scrap metals disposed of from industrial operations in the electrocoagulation process of PRWW under an optimum condition of 6.30 pH, current density of 22 mA/cm2 and exposure time of 39 min. The process obtained COD and phenol removal efficiencies of 91.18% and 91.46%, respectively.
Figure 7. Mechanism of electrocoagulation process for oil removal [73].
Electrooxidation (EO)
This is an advanced form of chemical oxidation technique which involves the generation of the oxidants that oxidize the pollutants through the application of electric current [40]. The EO process is sometimes considered as part of the AOPs from a broad perspective but only the oxidation process occurs on the surface of the anode electrode as opposed to direct oxidation in the latter [66]. The efficiency of the EO technique is affected by operating conditions such as the current density and electrode activity as well as the pollutants’ diffusion rate [40].3.4.3. Advanced Oxidation Processes
Chemical oxidation techniques are a set of treatment processes which can be broadly classified into two types: conventional chemical treatments and advanced oxidation processes [60]. Advanced oxidation processes are highly efficient techniques used in the treatment of different types of wastewater including petroleum industry wastewater, toxic effluents from pharmaceutical industry wastewaters, etc. In previous years, several works have been reported in the literature to examine the efficiency of these processes [60]. Precisely, advanced oxidation processes (AOPs) are a category of chemical treatment methods that produce free hydroxyl radical groups with strong oxidant potential which are capable of degrading contaminants. The most commonly employed AOPs in the treatment of PRWW include Fenton and photo-Fenton oxidation reaction processes, electrochemical oxidation, ozonation processes (O3), as well as heterogeneous photocatalytic oxidation [60][66]. The AOPs are gaining more attention nowadays as they are environmentally friendly techniques with less generation of secondary by-products and have shown high treatment efficiencies in the removal of organic compounds even at low concentrations [13]. The treatment capability is attributed to the strong hydroxyl radical (−OH) which has strong reactivity towards organic compounds and colour degradation potential [74]. Based on this, AOPs have been reported as an efficient treatment technology for the reduction of COD, odour, colour, and other specific pollutants as well as sludge treatment. It can also be used in integration with biological treatment processes as a non-selective integrated chemical oxidant with high efficiency in removing toxic organic compounds such as phenols.Fenton-Oxidation
Generally, among the AOP techniques, the Fenton technology is found to be very attractive due to its simplicity, high performance, low cost as well and lack of toxicity of the Fenton reagents which are usually ferrous ion and hydrogen peroxide [75]. The Fenton process (FP) is based on a redox reaction between a chemical mixture of hydrogen peroxide (H2O2) and ferric ions Fe2+ which have a strong oxidizing potential in an acid medium. It is a technique which was founded by Henry John Horstman Fenton in 1894 [65]. The hydroxyl radical is capable of the degradation of toxic and non-biodegradable pollutants by direct or indirect anodic oxidation [40]. The OH radicals are extremely strong reactive oxidizers with an oxidation potential of approximately, Eθ = 2.8 V and they are generally non-selective towards organic pollutants in wastewater [75]. There are two types of Fenton reactions: the standard Fenton reaction which is formed by a reaction between ferrous iron (Fe+2) ions and hydrogen peroxide (H2O2), as well as the Fenton-like reaction which is formed by a reaction of (Fe+3) ions and hydrogen peroxide [76]. The Fenton reaction conducted under intense light such as UV or sunlight which generates more hydroxyl radicals is called the photo-Fenton reaction. Normally the ratio between the iron ions and the peroxide which is [Fe2+]/[H2O2] is 1:2. However, the study reported by Quang et al. [77] suggested a ratio of 1:5 for a greater rate of degradation. The Fenton-oxidation technique has been widely investigated in the treatment of different types of wastewater effluents including textiles [77] and pharmaceuticals [78]. However, the Fenton process’s general limitations include the problem of adding H2O2 and its lower utilization and mineralization efficiencies [78]. The review reported by Elmobarak et al. [60] also summarized that the major drawbacks of the Fenton and the photo-Fenton processes include their requirement for a very low pH value of usually less than 2 as well as the need for the elimination of the iron ions after the reaction process. Additionally, the potentiality of the −OH radical degradation tends to reduce with a rise in the pH value. At a very low pH, there would be a creation of Fe (II) (H2O)2+ which can react with the hydrogen peroxide (H2O2) leading to reduced generation of hydroxyl radicals.Electro-Fenton Process
This is another novel oxidation technique which employs the electrochemical process and generation of TiO2 oxidants by the Fenton-oxidation process. This technique has been studied for the removal of COD, BOD, TPH, phenols and other recalcitrant compounds which are not easily degraded in conventional treatment plants [79]. For example, Fahim and Abbar [80] have reported a study treating Al-Dewaniya PRWW in Iraq by the electro-Fenton process using porous graphite electrodes as anode and cathode materials. They used a tubular type of electrochemical reactor with a cylindrical cathode made from porous graphite and a concentric porous graphite rod which acts as an anode. At a current density of 25 A/cm2, and operation time of 45 min with no addition of NaCl, the removal efficiency of COD was found to be 95.9% with an energy consumption of 8.921 kWh/kg per COD. The outcome of the experimental work has demonstrated the capability of the graphite–graphite electro-Fenton system as an effective technique in the removal of COD from petroleum wastewater. Similarly, Divyapriya and Nidheesh [81] also reported from their review that the use of graphene-based electrodes in the electro-Fenton technique is usually considered to be a promising and cleaner method to produce the reactive oxygen species that can rapidly mineralize organic contaminants.Photocatalysis
Photocatalysis is nowadays regarded as one of the most advanced, as well as environmentally friendly techniques for the total degradation of organic contaminants in various forms of wastewater [82]. A photo-catalyst is often defined as a material such as titanium oxide (TiO2) or transition metal oxides which can decompose harmful substances under the effect of sunlight containing UV rays [83]. The process occurs by the excitation of pairs of electrons in the valence band by UV which causes them to absorb higher energy than their band gap energy which then causes the simultaneous production of a hole in the valence band (h+) and an electron (e−) in the conduction band. Furthermore, the (h+) and (e−) species will then react with oxygen or water molecules to produce peroxide or hydroxyl radicals which are capable of degrading or decomposing organic compounds [13][83][84]. Depending on the specific characteristics of the semiconductor, the photolytic activity of photocatalysis is firstly initiated with the absorption of the energy in the form of photons which has an energy equal to or more than the band gap exhibited by the semiconductor such as the TiO2 [85]. The process works on the basis that the hole created on the catalysts would in turn generate highly reactive hydroxyl radicals with high reduction–oxidation potentials such as •O2−, H2O2, and •O2 that can play an important role in the photocatalytic reaction mechanism [86]. Photocatalysis has been employed as a more advanced practical and efficient process in the treatment of wastewater to degrade organic contaminants [75][84][86]. In achieving this, pore volume, pore structure, crystalline sizes, light intensity as well as specific surface area are the important parameters which determine the excellent performance of photocatalysts. Catalysts are the key requirement in the photocatalytic technique. A nano-catalyst usually possesses high surface area and density which gives it more photocatalytic activity and applicability in wastewater treatment [87][88]. For example, a titanium dioxide (TiO2)-based photocatalyst is the most widely used in wastewater treatment due to its high oxidizing ability of organic compounds, cost-effectiveness, nontoxicity, and environmental friendliness [84][89][90]. Graphene, which is a carbon-based material has also been tested for photocatalysis applications and demonstrated high potential applicability for general pollutant removal. Dang et al. [91] reported that the most important semiconductor catalyst widely employed for photocatalytic degradation of phenols includes: ZnO, CdS, TiO2, GaP, ZnS and Fe2O3.Properties of the Photocatalysts
Photocatalysts are usually employed either in the form of powders or thin films based on the requirements and scope of their application [92]. The nano forms of photocatalysts are better at fast reaction rates than their corresponding bulk forms due to their small size and high surface area [93]. However, using nanoparticles for wastewater treatment and pollutant degradation also has limitations related to their fast recombination losses and inadequate solar spectrum utilization [94].Metal Doping and Hybridization of Photocatalysts
Catalytic oxidation tends to increase with doping and hybridization modification processes, which increases the hydrophobicity and pollutant absorption strength of a photocatalyst [86]. To increase or intensify the photocatalytic activity of a catalyst, doping techniques are applied to improve sensitivity to UV light as well as reduce the band gap and recombination rate [84]. Besides improving photocatalytic activity, doping of catalysts also tends to reduce the amount of energy and wavelength required to be absorbed [84][86]. Metals such as iron [95], zinc [96], silver [97], platinum [98], or non-metal elements such as nitrogen [99] carbon and sulfur [100] have been employed as metallic dopants to enhance photocatalytic performance. On the other hand, catalyst hybridization is another technique used to enhance the degradation potential of organic pollutants where photocatalysts are sometimes combined with absorbents such as graphite, SiO2 and hydroxyapatite [101].Treatment of PRWW Using Photocatalysis
Due to the potential application using sunlight as a source of energy, this makes it an attractive technique for the degradation of organics from PRWW [87]. The degradation of organic contaminants from PRWW by photocatalysis has been widely investigated using various forms of photocatalyst under varying conditions. Ghasemi et al. [102] reported the treatment of PRWW by photocatalytic degradation using the TiO2/Fe-ZSM-5 photocatalyst with as-synthesized Fe-ZSM-5 zeolite produced via sol–gel method with a specific surface area of 304.6 m2/g and 29.28% loaded TiO2. About 80% COD removal was achieved at a pH level of 4, photocatalyst concentration of 2.1 g/L, and 45 °C UV exposure temperature through 240 min. Although high COD removal efficiency was achieved, the synthesis of ZSM-5 zeolite catalyst is associated with a high production cost via complex processes influenced by the effect of time and temperature [103]. Photocatalytic efficiencies of TiO2 and zeolite for the removal of COD and SO2− from PRWW were compared using a photocatalytic system by Tetteh et al. [104]. The operating conditions of the system include a catalyst dosage of 0.5–1.5 g/L, a mixing rate of 30–90 rpm and an 18 W UV light. After a reaction time of 15–45 min, the results show almost the same efficiency of 92% for zeolite and 91% for TiO2. Similarly, oil removal efficiency by photocatalysis has been examined by Mohammed et al. [105] using a ZnO photocatalyst under a solar light to determine the effects of dosage, pH and initial oil concentration. The outcome of the experiment shows 75% oil reduction, and the optimum catalyst concentration was found with a 3 g/L dosage of ZnO and a pH of 10. The oil degradation rate decreased with increasing oil concentration which might be due to an increased level of turbidity because of the oil suspension which in turn decreased the permeation of the solar light intensity.3.4.4. Combined H2O2/UV Advanced Oxidation Processes
The use of hydrogen peroxide as an oxidant in combination with potential sources of photon energy which can generate HO− radicals has been reported to be more successful in providing the hydroxyl radical that can degrade organic pollutants [80]. Using UV radiation of wavelengths >300 nm, H2O2 can decompose and generate HO− radicals [60]. Different researchers have reported that the degradation of pollutants by H2O2 continues steadily up to its highest efficiency after which it starts to decrease. This sudden decrease has been proven to be a result of the generating hydroxyl radicals which start to react with the additional H2O2 [106]. However, different advantages can be mentioned for the application of the H2O2/UV oxidation process, for example, there is no requirement for the removal of the hydroxyl radical after the treatment process and it is completely soluble in water [107].3.5. Integrated Treatment Processes (ITP)
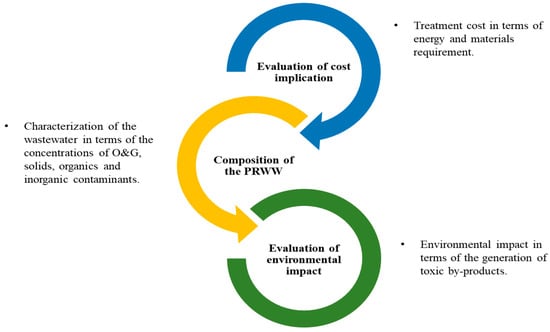
It can be noted that most conventional and advanced treatment techniques have specific limitations in terms of their efficiency and are sometimes associated with various demerits for the treatment of PRWW. Based on this there is always a key interest in developing a novel procedure that overcomes limitations such as the operational costs, treatment efficiency, and generation of secondary pollutants. These challenges can sometimes be addressed through an integrated or combined treatment process which can yield a better benefit. For example, the combination of AOP techniques integrated with conventional methods used for the treatment of different contaminated industrial wastewater has been confirmed to be more efficient. However, as indicated in Figure 8 the development of an integrated treatment process requires a good understanding of the PRWW characteristics, cost determination, as well as the requirements of environmental policies [60].
Figure 8. The basic considerations in the selection of an appropriate treatment technique.
In most cases, the development of an integrated treatment process is initiated from a combination of two or more conventional and advanced methods. Hence, the configurations of such a combination can be a two-step, three-step or even multiple-treatment process. This might comprise at least one conventional and one advanced, two conventional and one advanced, or even two advanced processes. For example, an advanced oxidation process integrated with biological or other treatment techniques increases the efficiency of degradation as well as the separation of the contaminants. Based on this, the biological processes can provide the needed decomposition of the residual oil and degradation etc. [108]. On the other hand, the integration of biological methods with membrane-based AOP techniques was also found to be an efficient process for the treatment of PRWW. However, in the development of a biological and chemical integrated process, the determination of the individual biological activity and chemical oxidation efficiencies is important for finding the optimal operating conditions for the combined process. This involves a profound knowledge of the operational conditions for both the biological and chemical processes.
4. Conclusions
Environmental pollution due to oil refinery wastewater is a global phenomenon that attracts serious attention due to its harmful effects on the ecosystem. Nowadays, the need to meet the maximum concentration limit for PRWW is usually challenging for petroleum refinery industries. This is because petroleum wastewater has a dynamic, complex nature. Hence, various conventional and advanced treatment techniques such as adsorption, membrane filtration, chemical precipitation, and biological systems have been designed to address this challenge over the years. While some already established techniques are mature in their applications, others are associated with various challenges and limitations. The appropriate treatment technology selection mostly depends on the oily wastewater composition, operational costs, efficiency, and environmental impacts. However, as the nature of PRWW is mainly in the form of oil-in-water emulsions, a correct understanding of their physical and chemical composition is needed. Although membrane treatment techniques have demonstrated an efficient removal capacity for organic and inorganic contaminants, they are associated with fouling and the problem of salt build-in bioreactors. New and advanced treatment techniques such as adsorption with modified non-conventional adsorbents, photocatalysis, and other advanced oxidation processes have been reported with significant efficiency in refinery wastewater treatment. For example, photocatalysis treatment techniques are effective in reducing COD, oil and grease concentrations, and phenol degradation. The alternative use of solar power as an energy source also makes it a considerable treatment option. Meanwhile, adsorption techniques using non-conventional adsorbents such as hydrogel were also effective in treating synthetic petroleum wastewater. They are less costly as well as environmentally friendly in their application.
References
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081.
- Salem, F.; Thiemann, T. Produced Water from Oil and Gas Exploration—Problems, Solutions and Opportunities. J. Water Resour. Prot. 2022, 14, 142–185.
- Al-Khalid, T.; El-Naas, M.H. Organic contaminants in refinery wastewater: Characterization and novel approaches for biotreatment. In Recent Insights in Petroleum Science and Engineering; InTechOpen: London, UK, 2018.
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2013, 10, S1913–S1922.
- Aljuboury, D.A.D.A.; Palaniandy, P.; Abdul Aziz, H.B.; Feroz, S. Treatment of petroleum wastewater by conventional and new technologies—A review. Glob. Nest J. 2017, 19, 439–452.
- Diya’uddeen, B.H.; Daud, W.M.A.W.; Aziz, A.A. Treatment technologies for petroleum refinery effluents: A review. Process Saf. Environ. Prot. 2011, 89, 95–105.
- Whale, G.; Hjort, M.; Di Paolo, C.; Redman, A.; Postma, J.; Legradi, J.; Leonards, P. Assessment of oil refinery wastewater and effluent integrating bioassays, mechanistic modelling and bioavailability evaluation. Chemosphere 2022, 287, 132146.
- Bayona, J.M.; Domínguez, C.; Albaigés, J. Analytical developments for oil spill fingerprinting. Trends Environ. Anal. Chem. 2015, 5, 26–34.
- Tang, X.; Eke, P.E.; Scholz, M.; Huang, S. Processes impacting on benzene removal in vertical-flow constructed wetlands. Bioresour. Technol. 2009, 100, 227–234.
- Pinzón-Espinosa, A. Unravelling the Chemistry behind the Toxicity of Oil Refining Effluents: From Characterisation to Treatment. Doctoral Dissertation, Brunel University: London, UK, 2018.
- Aziz, N.M.; Sabbar, A.A. Physiochemical Properties of Basrah oil refinery discharges and its potential effects on Shatt Al-Basrah Canal. Marsh Bull. 2013, 8, 39–57.
- Rahi, M.N.; Jaeel, A.J.; Abbas, A.J. Treatment of petroleum refinery effluents and wastewater in Iraq: A mini review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1058, 012072.
- Tetteh, E.K.; Rathilal, S. Evaluation of different polymeric coagulants for the treatment of oil refinery wastewater. Cogent Eng. 2020, 7, 1785756.
- Aljuboury, D.D.A.; Senthilkumar, R. Phenol degradation of industrial wastewater by photocatalysis. J. Innov. Eng. 2014, 2, 1–10.
- Radelyuk, I.; Tussupova, K.; Zhapargazinova, K.; Yelubay, M.; Persson, M. Pitfalls of wastewater treatment in oil refinery enterprises in kazakhstan—A system approach. Sustainability 2019, 11, 1618.
- Qaderi, F.; Abdolalian, S. Treatment of petroleum wastewater using solar power-based photocatalysis. In Petroleum Industry Wastewater: Advanced and Sustainable Treatment Methods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–170.
- Eldos, H.I.; Khan, M.; Zouari, N.; Saeed, S.; Al-Ghouti, M.A. Characterization and assessment of process water from oil and gas production: A case study of process wastewater in Qatar. Case Stud. Chem. Environ. Eng. 2022, 6, 100210.
- Petrowiki. Produced Oilfield Water. 2018. Available online: https://petrowiki.org/Produced_oilfield_water (accessed on 15 July 2019).
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of Petroleum oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064.
- El-Naas, M.H.; Abu Alhaija, M.; Al-Zuhair, S. Evaluation of a three-step process for the treatment of petroleum refinery wastewater. J. Environ. Chem. Eng. 2014, 2, 56–62.
- Tong, K.; Zhang, Y.; Liu, G.; Ye, Z.; Chu, P.K. Treatment of heavy oil wastewater by a conventional activated sludge process coupled with an immobilized biological filter. Int. Biodeterior. Biodegrad. 2013, 84, 65–71.
- Wang, Y.; Wang, Q.; Li, M.; Yang, Y.; He, W.; Yan, G.; Guo, S. An alternative anaerobic treatment process for treatment of heavy oil refinery wastewater containing polar organics. Biochem. Eng. J. 2015, 105, 44–51.
- Vendramel, S.; Bassin, J.P.; Dezotti, M.; Sant’Anna, G.L., Jr. Treatment of petroleum refinery wastewater containing heavily polluting substances in an aerobic submerged fixed-bed reactor. Environ. Technol. 2015, 36, 2052–2059.
- Wang, C.; Chen, Z.; Li, Y.; Feng, K.; Peng, Z.; Zhu, Y.; Yang, X. Refinery wastewater treatment via a multistage enhanced biochemical process. Sci. Rep. 2021, 11, 10282.
- Dehghani, M.; Alizadeh, M.H. The effects of the natural coagulant Moringa oleifera and alum in wastewater treatment at the Bandar Abbas Oil Refinery. Environ. Health Eng. Manag. 2016, 3, 225–230.
- Singh, B.; Kumar, P. Pre-treatment of petroleum refinery wastewater by coagulation and flocculation using mixed coagulant: Optimization of process parameters using response surface methodology (RSM). J. Water Process Eng. 2020, 36, 101317.
- Zueva, S.; Corradini, V.; Ruduka, E.; Veglio, F. Treatment of petroleum refinery wastewater by physicochemical methods. E3S Web Conf. 2020, 161, 01042.
- Ratman, I.; Kusworo, T.D.; Utomo, D.P.; Azizah, D.A.; Ayodyasena, W.A. Petroleum refinery wastewater treatment using three steps modified nanohybrid membrane coupled with ozonation as integrated pre-treatment. J. Environ. Chem. Eng. 2020, 8, 103978.
- Estrada-Arriaga, E.B.; Zepeda-Aviles, J.A.; García-Sánchez, L. Post-treatment of real oil refinery effluent with high concentrations of phenols using photo-ferrioxalate and Fenton’s reactions with membrane process step. Chem. Eng. J. 2016, 285, 508–516.
- Razavi, S.M.R.; Miri, T. A real petroleum refinery wastewater treatment using hollow fiber membrane bioreactor (HF-MBR). J. Water Process Eng. 2015, 8, 136–141.
- Nogueira, A.A.; Bassin, J.P.; Cerqueira, A.C.; Dezotti, M. Integration of biofiltration and advanced oxidation processes for tertiary treatment of an oil refinery wastewater aiming at water reuse. Environ. Sci. Pollut. Res. 2016, 23, 9730–9741.
- Coelho, A.; Castro, A.V.; Dezotti, M.; Sant’Anna, G.L., Jr. Treatment of petroleum refinery sourwater by advanced oxidation processes. J. Hazard. Mater. 2016, 137, 178–184.
- Chen, C.; Yoza, B.A.; Wang, Y.; Wang, P.; Li, Q.X.; Guo, S.; Yan, G. Catalytic ozonation of petroleum refinery wastewater utilizing Mn-Fe-Cu/Al2O3 catalyst. Environ. Sci. Pollut. Res. 2015, 22, 5552–5562.
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2013, 10, S1674–S1679.
- Zhang, Y.; Quan, X.; Chen, S.; Zhao, Y.; Yang, F. Microwave assisted catalytic wet air oxidation of H-acid in aqueous solution under the atmospheric pressure using activated carbon as catalyst. J. Hazard. Mater. 2006, 137, 534–540.
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 2021, 13, 980.
- Varjani, S.; Joshi, R.; Srivastava, V.K.; Ngo, H.H.; Guo, W. Treatment of Wastewater from Petroleum Industry: Current Practices and Perspectives. Environ. Sci. Pollut. Res. 2019, 27, 27172–27180.
- Shuokr, Q.A.; Sazan, M.A. Characteristics, treatment techniques, and operational limitations for refinery wastewater: Review. Reciklaza I Odrziv. Razvoj 2021, 14, 19–30.
- Jain, M.; Majumder, A.; Ghosal, P.S.; Gupta, A.K. A review on treatment of petroleum refinery and petrochemical plant wastewater: A special emphasis on constructed wetlands. J. Environ. Manag. 2020, 272, 111057.
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98.
- Renault, F.; Sancey, B.; Badot, P.-M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348.
- Van Le, T.; Imai, T.; Higuchi, T.; Yamamoto, K.; Sekine, M.; Doi, R.; Vo, H.T.; Wei, J. Performance of tiny microbubbles enhanced with “normal cyclone bubbles” in separation of fine oil-in-water emulsions. Chem. Eng. Sci. 2013, 94, 1–6.
- Iwuozor, K.O. Prospects and challenges of using coagulation-flocculation method in the treatment of effluents. Adv. J. Chem. A 2019, 2, 105–127.
- Turner, T.; Wheeler, R.; Stone, A.; Oliver, I. Potential alternative reuse pathways for water treatment residuals: Remaining barriers and questions—A review. Water Air Soil Pollut. 2019, 230, 227.
- Cai, L.; Zhang, Y.; Zhou, Y.; Zhang, X.; Ji, L.; Song, W.; Zhang, H.; Liu, J. Effective adsorption of diesel oil by crab-shell-derived biochar nanomaterials. Materials 2019, 12, 236.
- Gkika, D.A.; Mitropoulos, A.C.; Kyzas, G.Z. Why reuse spent adsorbents? The latest challenges and limitations. Sci. Total. Environ. 2022, 822, 153612.
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption removal of zinc (II) from the aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process Saf. Environ. Prot. 2016, 102, 336–352.
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36.
- Shenvi, S.S.; Isloor, A.M.; Ismail, A. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26.
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89.
- Moslehyani, A.; Ismail, F.; Othman, M.H.D.; Matsuura, T. Design and performance study of hybrid photocatalytic reactor-PVDF/MWCNT nanocomposite membrane system for treatment of petroleum refinery wastewater. Desalination 2015, 363, 99–111.
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066.
- Aziz, S.Q.; Fakhrey, E.S.A. The effect of kawergosk oil refinery wastewater on surrounding water resources. ZANCO J. Pure Appl. Sci. 2016, 28, 656–667.
- Hashemi, F.; Hashemi, H.; Dehghani, M.; Hoseini, M. Removal of heavy metals from oil refinery effluent by micellar-enhanced ultrafiltration (MEUF). J. Health Sci. Surveill. Syst. 2018, 6, 123–129.
- Lebron, Y.A.R.; Moser, P.B.; Moreira, V.R.; Silva, G.R.d.A.; Soalheiro, A.; de Souza, B.P.; de Paula, E.C.; Amaral, M.C.S. Osmotic membrane bioreactor (OMBR) in refinery wastewater treatment: The impact of a draw solute with lower diffusivity in the process performance. Chem. Eng. J. 2021, 406, 127074.
- Alnakeeb, A.; Rasheed, R.M. Chemical Precipitation method for Sulphate Removal from Treated Wastewater of Al-Doura Refinery. Eng. Technol. J. 2021, 39, 338–354.
- Altaş, L.; Büyükgüngör, H. Sulfide removal in petroleum refinery wastewater by chemical precipitation. J. Hazard. Mater. 2008, 153, 462–469.
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 102, 342–379.
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418.
- Elmobarak, W.F.; Hameed, B.H.; Almomani, F.; Abdullah, A.Z. A review on the treatment of petroleum refinery wastewater using advanced oxidation processes. Catalysts 2021, 11, 782.
- Ghimire, N.; Wang, S. Biological treatment of petrochemical wastewater. In Petroleum Chemicals: Recent Insight; IntechOpen: London, UK, 2018; pp. 55–74.
- Wu, C.; Zhou, Y.; Sun, Q.; Fu, L.; Xi, H.; Yu, Y.; Yu, R. Appling hydrolysis acidification-anoxic–oxic process in the treatment of petrochemical wastewater: From bench scale reactor to full scale wastewater treatment plant. J. Hazard. Mater. 2016, 309, 185–191.
- Vikrant, K.; Kim, K.-H. Nanomaterials for the adsorptive treatment of Hg (II) ions from water. Chem. Eng. J. 2018, 358, 264–282.
- Sabir, S. Approach of cost-effective adsorbents for oil removal from oily water. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1916–1945.
- Treviño-Reséndez, J.d.J.; Medel, A.; Meas, Y. Electrochemical technologies for treating petroleum industry wastewater. Curr. Opin. Electrochem. 2021, 27, 100690.
- Khalifa, O.; Banat, F.; Hasan, S.W. Electrochemical treatment of petroleum wastewater: Standalone and integrated processes. In Petroleum Industry Wastewater: Advanced and Sustainable Treatment Methods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 171–183.
- Ibrahim, M.H.; Banerjee, A.; El-Naas, M.H. Treatment of petroleum industry wastewater: Current practices and perspectives. In Petroleum Industry Wastewater: Advanced and Sustainable Treatment Methods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–6.
- Mickova, I.L. Advanced electrochemical technologies in wastewater treatment. Part II: Electro-flocculation and electro-flotation. Am. Acad. Sci. Res. J. Eng. Technol. Sci. 2015, 14, 273–294.
- Mohtashami, R.; Shang, J.Q. Electroflotation for treatment of industrial wastewaters: A focused review. Environ. Process. 2019, 6, 325–353.
- Zou, X.-L. Treatment of heavy oil wastewater by UASB–BAFs using the combination of yeast and bacteria. Environ. Technol. 2015, 36, 2381–2389.
- Merma, A.G.; Santos, B.F.; Rego, A.S.; Hacha, R.R.; Torem, M.L. Treatment of oily wastewater from mining industry using electrocoagulation: Fundamentals and process optimization. J. Mater. Res. Technol. 2020, 9, 15164–15176.
- Akkaya, G.K. Treatment of petroleum wastewater by electrocoagulation using scrap perforated (Fe-anode) and plate (Al and Fe-cathode) metals: Optimization of operating parameters by RSM. Chem. Eng. Res. Des. 2022, 187, 261–275.
- An, C.; Huang, G.; Yao, Y.; Zhao, S. Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci. Total. Environ. 2017, 579, 537–556.
- Zhang, E.; Li, W.; Gao, Y.; Lei, C.; Huang, H.-Y.; Yang, J.; Zhang, H.; Li, D. High-capacity reusable chitosan absorbent with a hydrogel-coated/aerogel-core structure and superhydrophilicity under oil for water removal from oil. ACS Appl. Bio Mater. 2020, 3, 5872–5879.
- Palaniandy, P.; Feroz, S. Advanced Oxidation Processes (AOPs) to Treat the Petroleum Wastewater. In Advanced Oxidation Processes (AOPs) in Water and Wastewater Treatment; IGI Global: Hershey, PA, USA, 2019; pp. 99–122.
- Nidheesh, P.V.; Gandhimathi, R. Textile Wastewater Treatment by Electro-Fenton Process in Batch and Continuous Modes. J. Hazard. Toxic Radioact. Waste 2015, 19, 04014038.
- Quang, H.H.P.; Dinh, N.T.; Thi, T.N.T.; Bao, L.T.N.; Yuvakkumar, R.; Nguyen, V.-H. Fe2+, Fe3+, Co2+ as highly efficient cocatalysts in the homogeneous electro-Fenton process for enhanced treatment of real pharmaceutical wastewater. J. Water Process Eng. 2022, 46, 102635.
- Giwa, A.-R.A.; Bello, I.A.; Olabintan, A.B.; Bello, O.S.; Saleh, T.A. Kinetic and thermodynamic studies of fenton oxidative decolorization of methylene blue. Heliyon 2020, 6, e04454.
- Kulkarni, S.J.; Goswami, A.K. A review on wastewater treatment for petroleum industries and refineries. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 1, 280–283.
- Fahim, A.S.; Abbar, A.H. Treatment of petroleum refinery wastewater by electro-Fenton process using porous graphite electrodes. Egypt. J. Chem. 2020.
- Divyapriya, G.; Nidheesh, P.V. Importance of graphene in the electro-fenton process. ACS Omega 2020, 5, 4725–4732.
- Rashid, J.; Barakat, M.A.; Ruzmanova, Y.; Chianese, A. Fe3O4/SiO2/TiO2 nanoparticles for photocatalytic degradation of 2-chlorophenol in simulated wastewater. Environ. Sci. Pollut. Res. 2015, 22, 3149–3157.
- Sakka, S. Chapter 11.1.2. Sol–Gel Process and Applications. In Handbook of Advanced Ceramics; Elsevier: Amsterdam, The Netherlands, 2013.
- Lau, W.-J.; Ismail, A.F.; Isloor, A.; Al-Ahmed, A. Advanced Nanomaterials for Membrane Synthesis and Its Applications; Elsevier: Amsterdam, The Netherlands, 2019.
- Abeish, A.B.M.S. Enhanced Photocatalytic Degradation of Biorefractory Pollutants in Petroleum Refinery Wastewater. Doctoral Dissertation, Curtin University, Bentley, Australia, 2015.
- Park, S.J.; Yang, J.H.; Joo, M.H.; Sohn, Y. Current status, research gaps, and future scope for nanomaterials toward visible light photocatalysis. In Nanostructured Materials for Visible Light Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2022; pp. 569–608.
- Sushma; Yadav, A. Biological and physicochemical combination processes. In Nanomaterials for Air Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 361–372.
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248.
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO2)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262.
- Stasinakis, A.S. Use of selected advanced oxidation processes (AOPs) for wastewater treatment—A mini review. Glob. NEST J. 2008, 10, 376–385.
- Dang, T.T.T.; Le, S.T.T.; Channei, D.; Khanitchaidecha, W.; Nakaruk, A. Photodegradation mechanisms of phenol in the photocatalytic process. Res. Chem. Intermed. 2016, 42, 5961–5974.
- Baradaran, M.; Ghodsi, F.E. Investigation of the properties of oxide-based multilayer thin films and their use in the photocatalytic applications. In Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 697–715.
- Tahir, M.B.; Sohaib, M.; Sagir, M.; Rafique, M.D. Role of nanotechnology in photocatalysis. Encycl. Smart Mater. 2020, 2, 578–589.
- Das, S.; Dhara, S. Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Elsevier: Amsterdam, The Netherlands, 2021.
- Shymanovska, V.; Khalyavka, T.; Manuilov, E.; Gavrilko, T.; Aho, A.; Naumov, V.; Shcherban, N. Effect of surface doping of TiO2 powders with Fe ions on the structural, optical and photocatalytic properties of anatase and rutile. J. Phys. Chem. Solids 2021, 160, 110308.
- Xu, J.-C.; Shi, Y.-L.; Huang, J.-E.; Wang, B.; Li, H.-L. Doping metal ions only onto the catalyst surface. J. Mol. Catal. A Chem. 2004, 219, 351–355.
- Sahoo, C.; Gupta, A.K.; Pillai, I.M.S. Photocatalytic degradation of methylene blue dye from aqueous solution using silver ion-doped TiO2 and its application to the degradation of real textile wastewater. J. Environ. Sci. Health Part A 2012, 47, 1428–1438.
- Hu, Y.; Song, X.; Jiang, S.; Wei, C. Enhanced photocatalytic activity of Pt-doped TiO2 for NOx oxidation both under UV and visible light irradiation: A synergistic effect of lattice Pt4+ and surface PtO. Chem. Eng. J. 2015, 274, 102–112.
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2390.
- Saleh, T.A. Polymer Hybrid Materials and Nanocomposites: Fundamentals and Applications; William Andrew: Oxford, UK, 2021.
- Hamal, D.B.; Klabunde, K.J. Synthesis, characterization, and visible light activity of new nanoparticle photocatalysts based on silver, carbon, and sulfur-doped TiO2. J. Colloid Interface Sci. 2007, 311, 514–522.
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Preparation, characterization and photocatalytic application of TiO2/Fe-ZSM-5 nanocomposite for the treatment of petroleum refinery wastewater: Optimization of process parameters by response surface methodology. Chemosphere 2016, 159, 552–564.
- Widayat, W.; Annisa, A.N. Synthesis and characterization of ZSM-5 catalyst at different temperatures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 214, 012032.
- Tetteh, E.K.; Obotey Ezugbe, E.; Rathilal, S.; Asante-Sackey, D. Removal of COD and SO42− from oil refinery wastewater using a photo-catalytic system—Comparing TiO2 and zeolite efficiencies. Water 2020, 12, 214.
- Mohammed, S.A.; Al-Azawiey, S.S.; Ali, A.H. Treatment of Organic Compounds Resulting from Oil Refineries under Solar Light and Reuse it for Industrial Purpose. Muthanna J. Eng. Technol. 2021, 9, 20–24.
- Huang, Y.; Kong, M.; Coffin, S.; Cochran, K.H.; Westerman, D.C.; Schlenk, D.; Richardson, S.D.; Lei, L.; Dionysiou, D.D. Degradation of contaminants of emerging concern by UV/H2O2 for water reuse: Kinetics, mechanisms, and cytotoxicity analysis. Water Res. 2020, 174, 115587.
- Dhivakar, V.; Rajan, T. BTEX compounds removal from waste water by using UV&UV/H2O2 process. Int. J. Recent Eng. Sci. 2018, 5, 22–25.
- Oller, I.; Malato, S.; Sánchez Pérez, J.A. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166.
More
