Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Xianyi Sha and Version 4 by Fanny Huang.
Airway mucus is a complex viscoelastic gel mainly composed of water, glycoproteins, lipids, enzymes, minerals, etc. Among them, glycoprotein is the main factor determining mucus-gel-like rheology. Airway mucus forms a protective barrier by secreting mucin, which represents the absorption barrier, especially for more lipophilic drugs. It rapidly clears the drug from the airways through physiological mucus clearance mechanisms, so the drug does not remain in the lungs or reach the airway epithelial tissue for a long time.气道粘液是一种复杂的粘弹性凝胶,主要由水、糖蛋白、脂质、酶、矿物质等组成。其中,糖蛋白是决定粘液凝胶样流变性的主要因素。气道粘液通过分泌粘蛋白形成保护屏障,粘蛋白代表吸收屏障,特别是对于更亲脂性的药物。它通过生理性粘液清除机制迅速从气道中清除药物,因此药物不会长时间留在肺部或到达气道上皮组织。
- airway mucus
- nanoparticles
1. Introduction简介
The mucus layer plays a vital role in human health, as it is the front line of the body's defense system [1], capable of selectively penetrating foreign bodies and pathogens, thereby protecting the normal functioning of the organism [2]. Lung mucus consists of two layers: the fluid layer on the airway surface and the layer around the eyelashes. The former consists of gel-forming mucus and is responsible for adsorbing and encapsulating inhalation particles. The latter is where surface cells beat and relax, efficiently transporting the mucus layer to the outside of the lungs [3][4]. The composition and thickness of the mucus layer is not constant粘液层在人体健康中起着至关重要的作用,因为它是人体防御系统的前线[1],能够选择性地渗透异物和病原体,从而保护生物体的正常功能[2]。肺粘液包括两层:气道表面的液体层和睫毛周围层。前者由凝胶形成粘液组成,负责吸附和封装吸入颗粒。后者是表面细胞跳动和放松的地方,有效地将粘液层输送到肺部外部[3,4]。粘液层的组成和厚度不是恒定的; It is a dynamic system whose composition and thickness are caused by the continuous secretion and clearance of mucus [5].它是一个动态系统,其组成和厚度是由粘液的持续分泌和清除引起的[5]。
In addition to water, the main component of mucus is mucin, which can be divided into two subtypes according to its glycosylation variability: secreted mucin and membrane-bound mucin. Membrane-binding proteins bind mainly to the surface of the mucosal epithelium, while the disulfide bonds between them link the secreted mucins to form a continuous gel state [6]. Given the importance of lung mucus to the bioavailability of pulmonary drugs, studying the important components and tissues of lung mucus [5] is critical to understanding its barrier function. Drugs delivered directly to the airways or inhalation therapy [7] are commonly used to treat lung disease. Among them, inhalation therapy makes it easier to deliver therapeutic drugs to the pulmonary mucosa, so it is possible to significantly reduce the dose of the drug and further reduce its side effects. However, due to the inherent clearance mechanism of the pulmonary mucosa, the drug is less bioavailable in the lungs, and most of the drug is cleared in the pulmonary mucosa [8][9].除水外,粘液的主要成分是粘蛋白,根据其糖基化的可变性可分为两种亚型:分泌粘蛋白和膜结合粘蛋白。膜结合蛋白主要与粘膜上皮表面结合,而它们之间的二硫键连接分泌的粘蛋白形成连续的凝胶状态[6]。鉴于肺粘液对肺部药物生物利用度的重要性,研究肺粘液的重要成分和组织[5]对于了解其屏障功能至关重要。药物直接输送到气道或吸入疗法[7]通常用于治疗肺部疾病。其中,吸入疗法更容易将治疗药物输送到肺粘膜,因此可以显着减少药物的剂量并进一步减少其副作用。然而,由于肺黏膜固有的清除机制,该药物在肺部的生物利用度较低,大部分药物在肺黏膜中清除[8,9]。
Over the past few decades, convincing data have confirmed that nanodelivery systems can be promising carriers for delivering drugs through the mucus layer, including polymer nanoparticles, liposomes, polymer micelles, and nanoparticles [10][11][12][13][14][15]. After inhalation delivery to the airway, these nanoparticles can penetrate or remain in the airway mucus layer by osmosis-mediated, adhesion-mediated, and biomimetic-mediated, respectively [16][17][18]. The benefits of utilizing nanocarriers are increased bioavailability of the drug and reduced unwanted toxicity due to its surface modification, suitable nanometer size, and blood stability [19][20].在过去的几十年中,令人信服的数据已经证实,纳米递送系统可以成为递送药物穿透粘液层的有希望的载体,包括聚合物纳米颗粒、脂质体、聚合物胶束和纳米颗粒[10,11,12,13,14,15]。药物通过吸入输送到气道后,这些纳米颗粒可分别通过渗透介导、粘附介导和仿生介导的方法穿透或保留在气道黏液层中[16,17,18]。利用纳米载体的好处是提高了药物的生物利用度,并由于其表面修饰、合适的纳米尺寸和血液稳定性而降低了不必要的毒性[19,20]。
2. Pathophysiology of Airway Mucus气道黏液的病理生理学
According据统计,一个典型的成年人在平静的状态下每分钟呼吸大约 to statistics, a typical adult breaths about 16-20 times per minute in a calm state. The amount of gas inhaled or exhaled is approximately 500 mL, or tidal volume [21]. As a result, the surface of the human airways constantly interacts with the external environment, including inhaled particles and pathogens. The mucociliary clearance mechanism is an important defense mechanism for maintaining a normal physiological state of the human airway [22]. The mucocilia removal system avoids the retention of pathogenic bacteria by constantly renewing the mucus blanket while removing pathogenic microorganisms and inhaled particles. The mucosal cilia clearance system [23] has three main components: the surface fluid layer in contact with the airway lumen, the periciliary fluid layer that supports cilia beating, and the respiratory epithelium composed of secretory cells [24] (Figure 次。每次吸入或呼出的气体量约为500mL,即潮气量[21]。因此,人体气道表面不断与外部环境相互作用,包括吸入的颗粒和病原体。黏液纤毛清除机制是人体气道维持正常生理状态的重要防御机制[22]。粘液纤毛清除系统通过不断更新粘液毯,同时去除病原微生物和吸入的颗粒物,避免病原菌的滞留。黏膜纤毛清除系统[23]有三个主要组成部分:与气道腔接触的表面液层、支持纤毛跳动的纤毛周围液层和由分泌细胞组成的呼吸道上皮[24](图1).)。
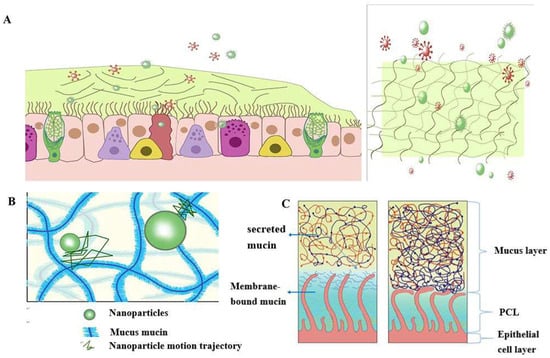
Figure 图1Schematic.粘液屏障的结构和功能示意图: diagram of the structure and function of the mucus barrier: ((A) The mucosal cilia removal system has three main components: the surface fluid layer in contact with the airway lumen, the periciliary fluid layer that supports cilia beating, and the respiratory epithelium composed of secretory cells. Constant renewal of the mucus blanket avoids the retention of pathogenic bacteria, while removing pathogenic microorganisms (red spheres) and inhaled particulate matter (green spheres))粘膜纤毛清除系统有三个主要组成部分:与气道腔接触的表面液层、支持纤毛跳动的纤毛周液层和由分泌细胞组成的呼吸道上皮。不断更新粘液毯可避免病原菌的滞留,同时去除病原微生物(红色球体)和吸入的颗粒物(绿色球体); ((B) Schematic diagram of the nanoparticle structure through the mucin network. Small molecules can cross the mucus barrier by diffusion freely, but most large molecules do not easily cross the mucus barrier)通过粘蛋白网络的纳米颗粒结构示意图。小分子可以通过自由扩散穿过粘液屏障,但大多数大分子不容易穿过粘液屏障; ((C) Schematic diagram of )正常情况下由于粘液和PCL collapse under normal conditions due to mucus and PCL layers and airway dehydration.层以及气道脱水而导致PCL塌陷的示意图。
The mucus layer is one of the key components of the mucocilia removal system, which acts as both a physical and a chemical barrier. The mucus layer consists of hundreds of substances and contains 粘液层是粘液纤毛清除系统的关键组成部分之一,它既是物理屏障,也是化学屏障。粘液层由数百种物质组成,含有98% water and 2% solids [25]. The main macromolecule of this 的水和2%的固体[25]。这种2% solid substance is mucin, a macromolecule formed from a family of glycoproteins that are highly glycosylated [26][27][28]. The main secreted mucins in the airways are 固体物质的主要大分子是粘蛋白,这是一种由高度糖基化的糖蛋白家族形成的大分子[26,27,28]。气道中主要的分泌粘蛋白是MUC5B and 和MUC5AC [29][30], which have characteristic domains formed by repeated tandem associations of abundant proline, serine, serine, and threonine. The repeat sequence undergoes [29,30],它们具有由丰富的脯氨酸、丝氨酸、丝氨酸和苏氨酸的重复串联关联形成的特征性结构域。重复序列经历O-glycosylation to harden the mucin backbone and increase the stiffness of the mucin chain, thus maintaining the gel morphology of the mucus. Mucin itself can bind to therapeutic drugs in a non-specific way. 糖基化以使粘蛋白骨架变硬并增加粘蛋白链的刚度,从而保持粘液的凝胶形态。粘蛋白本身可以以非特异性方式与治疗药物结合。Pavan G. Bhat and colleagues studied the permeability of porcine gastric mucus to five substances: isoniazid [31], pentamidine [32], rifampicin [33], p-aminosalicylic acid [34], and pyrazinamide [35], all of which can be delivered to lung targets using inhalation therapy. The permeability of all two agents was significantly reduced in the presence of mucus compared with the permeability of the blank buffer [2]. The above results show that all compounds bind specifically to mucin molecules before passing through the mucus layer, resulting in reduced penetration through the mucus layer. The pore size of the mucus layer also plays a crucial role in the penetration of the drug. Anionic and nonionic surfactants have a more significant effect on the mucus permeability of nanoparticles and their mucus barrier modulation ability, which also depends on the type of surfactant. 及其同事研究了猪胃粘液对五种物质的渗透性:异烟肼[31],喷他脒[32],利福平[33],对氨基水杨酸[34]和吡嗪酰胺[35],所有这些都可以使用吸入疗法输送到肺部目标。与空白缓冲液的渗透性相比,所有2种药物在存在黏液时的渗透性均显著降低[80]。上述结果表明,所有化合物在穿过粘液层之前都与粘蛋白分子特异性结合,导致它们通过粘液层的渗透减少。粘液层的孔径在药物的渗透中也起着至关重要的作用。阴离子和非离子表面活性剂对纳米颗粒粘液渗透性及其粘液屏障调节能力有更显著的影响,粘液屏障调节能力也取决于表面活性剂的类型。十二烷基硫酸钠(Sodium lauryl sulfate (SDS) increased the composite viscosity and viscoelasticity of mucus, but poloxamer showed a downward trend. Tween 80 largely retains its original mucus rheological and morphological properties and may be a promising candidate for promoting the penetration of nanoparticles into the mucus barrier with good safety. Studies have shown that some small molecules can cross the mucus barrier through free diffusion, but most large molecules do not easily cross the mucus barrier [27]. Therefore, the permeability of the mucus layer may be limited by its pore size can be infered [36].)增加了粘液的复合黏度和黏弹性,但泊洛沙姆呈下降趋势。吐温27在很大程度上保留了原始的粘液流变学和形态学特性,并且可能是促进纳米颗粒渗透粘液屏障的有希望的候选者,具有良好的安全性。研究表明,一些小分子可以通过自由扩散穿过粘液屏障,但大多数大分子不容易穿过粘液屏障[36]。因此,我们可以推断粘液层的渗透性可能受到其孔径的限制[<>]。
The periciliated liquid layer (纤毛周围液体层(PCL) is ideal for cilia flow and is approximately 5-10 μm thick, [4] corresponding to the length of the cilia. If the layer is too thick, cilia cannot reach the upper mucus layer and therefore cannot perform their clearing defense function. At the same time, if this layer is too thin, the upper mucus layer adheres to the cilia and blocks their movement [23]. Hydration of airway surface fluids is critical to achieving mean mucus clearance [25])是纤毛流动的理想选择,厚度约为5-10μm,[4]对应于纤毛的长度。如果层太厚,纤毛无法到达上粘液层,因此无法发挥其清除防御功能。同时,如果该层太薄,上粘液层会粘附在纤毛上并阻止其运动[23]。气道表面液体的水合作用对于实现平均黏液清除率至关重要[25]; In the normal airway [37], water is distributed between the mucus layer and the periciliary fluid layer, and the layer with low osmotic pressure changes its concentration more easily than the layer with high osmotic pressure. 在正常气道中[37],水分布在粘液层和睫周液层之间,渗透压低的层比渗透压高的层更容易改变其浓度。Button et al. propose a brush gel model that shows that when the mucus layer is heavily hydrated [4], its osmotic pressure drops sharply, so that the liquid from the airway surface enters the mucus layer first, while the 等人提出了一种刷式凝胶模型,该模型表明,当粘液层大量水合时[4],其渗透压急剧下降,因此来自气道表面的液体首先进入粘液层,而PCL remains unchanged. Conversely, when airway dehydration occurs, the mucus layer is dehydrated first, increasing its concentration, increasing the osmotic pressure of the mucus layer, and the 保持不变。反之,当气道脱水发生时,粘液层首先脱水,增加其浓度,增加了粘液层的渗透压,PCL layer is compressed under high pressure, causing the PCL to collapse. PCL is a network structure composed of various macromolecular substances, the size of which is not constant between grids, related to the height of the PCL layer [38]. When the airway surface fluid is overhydrated, resulting in the collapse of the 层在高压下被压缩,导致PCL塌陷。PCL是由各种大分子物质组成的网状结构,其大小在网格之间不是恒定的,与PCL层的高度有关[38]。当气道表面液体发生过度水合作用,导致PCL layer, the mesh size of the 层塌陷时,PCL layer is subsequently reduced; Assuming that the drug particles reach the mucus layer, the penetration of the drug particles in the lung mucosa is significantly reduced due to the reduction of the pores in the PCL grid. In addition, a series of changes in the composition and structure of the mucus layer in the pathological state, such as an imbalance in ion transport in the lung airways in patients with cystic fibrosis (CF), leads to a decrease in the volume of fluid on the surface of the airway, a significant increase in the viscoelasticity of the mucus, and impaired clearance of mucocilia [39][40][41][42]. In addition, under pathological conditions, the concentration of mucin, 层的网格尺寸随后减小;假设药物颗粒到达粘液层,由于PCL网格空隙的减少,药物颗粒在肺粘膜中的渗透显着降低。此外,粘液层的组成和结构在病理状态下发生一系列变化,例如囊性纤维化(CF)患者肺气道中离子转运的不平衡,导致气道表面液体体积减少,粘液粘弹性显着增加,粘液纤毛清除受损[39,40,41,42]。此外,在病理条件下,粘蛋白、DNA, and actin increased significantly, significantly reducing the average size and size distribution of the mesh spacing, thereby severely hindering the transport of nanoparticles. Therefore, there is an urgent need for nanodelivery carriers capable of carrying drugs through dense mucus layers.和肌动蛋白的浓度显著增加,显著减小了网间距的平均尺寸和尺寸分布,从而严重阻碍了纳米颗粒的运输。因此,迫切需要能够通过致密粘液层携带药物的纳米递送载体。
3. Nanoparticle-Mediated Effective Enhancement of Drug Retention and Penetration in the Airway Mucosa纳米颗粒介导的有效增强药物在气道粘膜中的滞留和渗透
Based on the barrier properties of airway mucus described earlier, researchers can design different strategies to enhance the penetration of drugs in the pulmonary mucosa according to their characteristics. Among them, nanoparticle formulations have significant advantages in improving cell penetration and therefore may be a promising approach to treating lung diseases [43]. However, when used as a transport carrier, it faces a double barrier of size filtration of the mucus layer and interaction filtration. Since the barrier properties of mucus change its behavior, it is necessary to design nanoparticles appropriately, such as changing the surface properties of nanoparticles (including particle surface functional groups and charge density, etc.), changing their particle size [44], etc. In addition, enlarging mucosal lattice voids by disrupting specific non-covalent interactions of mucogel is also an effective way to promote mucosal drug penetration. Table 基于前面描述的气道粘液的屏障特性,我们可以根据其特性设计不同的策略来增强药物在肺粘膜中的渗透。其中,纳米颗粒制剂在改善细胞渗透方面具有显着优势,因此可能是治疗肺部疾病的有前途的方法[43]。然而,当用作运输载体时,它面临着粘液层的尺寸过滤和相互作用过滤的双重屏障。由于粘液的屏障特性会改变其行为,因此需要对纳米颗粒进行合适的设计,例如改变纳米颗粒的表面性质(包括颗粒表面官能团和电荷密度等),改变其粒径[44]等。此外,通过破坏粘液凝胶的特定非共价相互作用来扩大粘膜晶格空隙也是促进粘膜药物渗透的有效方法。表1 summarizes the various types of nanoparticles that enhance mucus penetration and retention.总结了增强粘液渗透和保留的各种类型的纳米颗粒。
Table
表 1.
Summary of nanoparticles for enhanced mucus penetration and retention.
用于增强粘液渗透和保留的纳米颗粒摘要。
| 纳米颗粒类型 | 配方详情 | 结果 | 参考 |
|---|---|---|---|
|
|
|
[45] |
|
|
[46] | |
|
|
[47] | |
|
|
|
[48] |
|
|
[1] | |
|
|
|
[49] |
|
|
[50] | |
|
|
[51] | |
|
|
|
[52] |
|
|
[53] | |
|
|
|
[54] |
|
|
[55] | |
|
|
[56] | |
|
|
|
[57] |
|
|
[58] | |
|
|
|
[59] |
| Nanoparticle type | Recipe details | outcome | reference |
|---|---|---|---|
|
|
|
[45] |
|
|
[46] | |
|
|
[47] | |
|
|
|
[48] |
|
|
[1] | |
|
|
|
[49] |
|
|
[50] | |
|
|
[51] | |
|
|
|
[52] |
|
|
[53] | |
|
|
|
[54] |
|
|
[55] | |
|
|
[56] | |
|
|
|
[57] |
|
|
[58] | |
|
|
|
[59] |
