Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by Mirela Ivancic Santek.
Bioethanol is the most widely used alternative transportation fuel to petrol. Bioethanol is considered a clean, renewable, and environmentally friendly fuel that can contribute to climate change mitigation, decreased environmental pollution, and enhanced energy security.
- biofuels
- bioethanol
- lignocellulosic biomass
1. Introduction
The production of biofuels is of rapidly growing interest for several reasons, including energy independence, reducing reliance on fossil fuels, the development of rural regions, and a reduction in greenhouse gas emissions [94][1]. The annual production of biofuels globally increased from 139.4 in 2016 to 174.9 billion liters in 2022, with an incline of 8% due to the COVID-19 pandemic in 2020. The leading producers of biofuels are still the USA, with 57.5 and 14.5 billion liters of bioethanol and biodiesel, respectively, followed by Brazil, with 35.6 billion liters of bioethanol. Still, most of the ethanol is produced from sugar-based feedstocks, sugarcane in Brazil, and corn in the United States of America (USA), while the production of lignocellulosic bioethanol is negligible (<0.2 of total annual production) [95][2]. Bioethanol is a widely used biofuel for transportation and is available in different blends with gasoline for use in conventional and flexible fuel vehicles. Nowadays, most vehicles use E10 and E15 blends with 10 and 15% ethanol, respectively. In comparison, flexible fuel vehicles run on the blend E85, which contains from 51% to 83% ethanol, depending on geography and the season, and pure hydrated ethanol (93% ethanol; 7% water) [94,96][1][3]. Nowadays, bioethanol is commonly produced in bio-refineries, which integrate several processes, generating multiple products, including energy, various chemicals, and biomaterials derived from feedstock/s to enhance the sustainability and economic competitiveness of production and reduce environmental concerns.
2. Bio-Refineries
A bio-refinery is a facility for sustainable biomass processing into food/feed ingredients, chemicals, materials, and bioenergy in an environmentally sound, socially acceptable, and cost-competitive manner. The most commonly used biomass is classified into four groups based on origin:
- (1)
-
Energy crops: herbaceous energy crops (e.g., switchgrass, miscanthus, bamboo, AND sweet sorghum), woody energy crops (e.g., hybrid poplar, hybrid willow, silver maple, AND eastern cottonwood), agricultural crops (oil crops, e.g., jatropha, oilseed rape, sunflower, castor oil, palm, and coconut; cereals, e.g., barley, wheat, oats, maize, and rye; and sugar and starchy crops, e.g., sweet sorghum, potato, and sugarcane), and aquatic crops (e.g., giant kelp, other seaweed, and microalga);
- (2)
-
Agricultural residues and waste: agricultural residues (e.g., sugar cane bagasse, corn stover, cobs, stalks and leaves, wheat straw, rice straw, rice hulls, nut hulls, and barley straw) and by-products and waste (wood processing by-products, e.g., sawdust, bark, branches, and leaves/needles; animal manure);
- (3)
A similar concept used in bio-refineries has been applied for decades in the food processing industry to produce starch from potatoes, wheat, and corn, crystalline sugar, wine, beer, and vegetable oil, as well as in pulp and paper production. A range of marketable products has been developed from intermediate or final products as well as waste streams such as feed, materials, biomaterials, energy, chemicals, etc. The production of fossil fuels from crude oil in convectional petroleum refineries is based on a comparable concept. In addition to fuels for transport, petroleum refineries generate electricity and various high-value chemicals using optimized and mature technology, creating additional income [97][5].
Several classification systems of biorefineries are described in the literature based on the source of feedstock, technological implementation status and biomass conversion route used, and product(s) targeted [97][5]. The commonly used classification is based on the feedstock. First-generation biorefineries utilize cereal (e.g., wheat, corn, rice, and barley), edible oilseed crops (e.g., rapeseed, sunflower, and soybeans), and sugar crops (e.g., sugar beet and sugarcane). Most of the currently produced biofuels are classified as first generation. The production of biofuels in second-generation bio-refineries relies on non-edible feedstocks such as lignocellulosic biomass (agriculture and forest residues such as corn stover and cobs and wheat straw), food waste, and energy crops like Jerusalem artichoke, Miscanthus x giganteus, millet, and jatropha. In third-generation biorefineries, phototrophically grown algal and microalgal biomass is processed into biofuels and other value-added products [98,99][6][7].
Feedstock for the production of biofuels greatly affects the economic and environmental sustainability of the process. The production costs for biofuels produced from edible crops (first-generation biofuels) are significantly lower compared to biofuels produced from lignocellulosic biomass and waste (second-generation biofuels), photographically grown algae (third-generation biofuel), or genetically modified microorganisms and crops (fourth-generation biofuel). In 2018, the price of bioethanol produced from sugarcane was USD 0.56/L, while the price of bioethanol produced from sugarcane bagasse was more than two times higher (USD 1.33/L) [100][8]. The production of advanced biofuels still strongly depends on subsidies and other market interventions to compete economically with fossil fuels. Despite limitations related to the cost-effectiveness in scaling to the commercial level, second- to fourth-generation biofuels have greater potential to reduce greenhouse gas emissions than first-generation biofuels. The environmental and economic feasibility of first-generation biofuel production is questionable since it depends on edible crops that compete with food production for the same feedstock and requires arable land, fertilizers, and water for plant growth. Moreover, increased feedstock demand leads to land use change and deforestation, accelerating climate change and biodiversity loss [98][6].
3. Strategies for Bioethanol Production
Two major routes for converting lignocellulosic biomass to biofuels are (1) biochemical and (2) thermochemical (Figure 1). Gasification, pyrolysis, and hydrothermal liquefaction are extensively used thermochemical methods for the conversion of lignocellulosic biomass. The main products of thermochemical conversion are gases (gasification) and liquids (pyrolysis and hydrothermal liquefaction), which are processed into biofuels and other valuable chemicals. Gasification includes the thermal decomposition of biomass at high temperatures (>800 °C) followed by a partial oxidation process to produce a mixture of gases consisting mainly of carbon monoxide and hydrogen, called synthetic gas or syngas, which are further used for the production of mixed alcohols or Fischer–Tropsch hydrocarbons via catalytic reaction [101,102][9][10]. Pyrolysis also involves the thermal decomposition of lignocellulosic biomass, which is conducted at lower temperatures (400–600 °C) in the absence of oxygen. Lignocellulosic biomass is converted into liquid bio-oil, solid biochar, and non-condensable gases rich in CO, CH4, and H2. After purification, bio-oil is used as feedstock for the production of different biofuels [103][11]. Hydrothermal liquefaction, also known as hydrous pyrolysis, involves the slow thermal decomposition of biomass under pressure from 4 to 22 MPa and temperatures lower than other thermochemical conversion processes (temperature: 250–400 °C). Hydrothermal liquefaction is less energy-intensive and more economically feasible due to lower operating temperatures and the omission of the drying step needed in other thermochemical conversion processes [104][12].
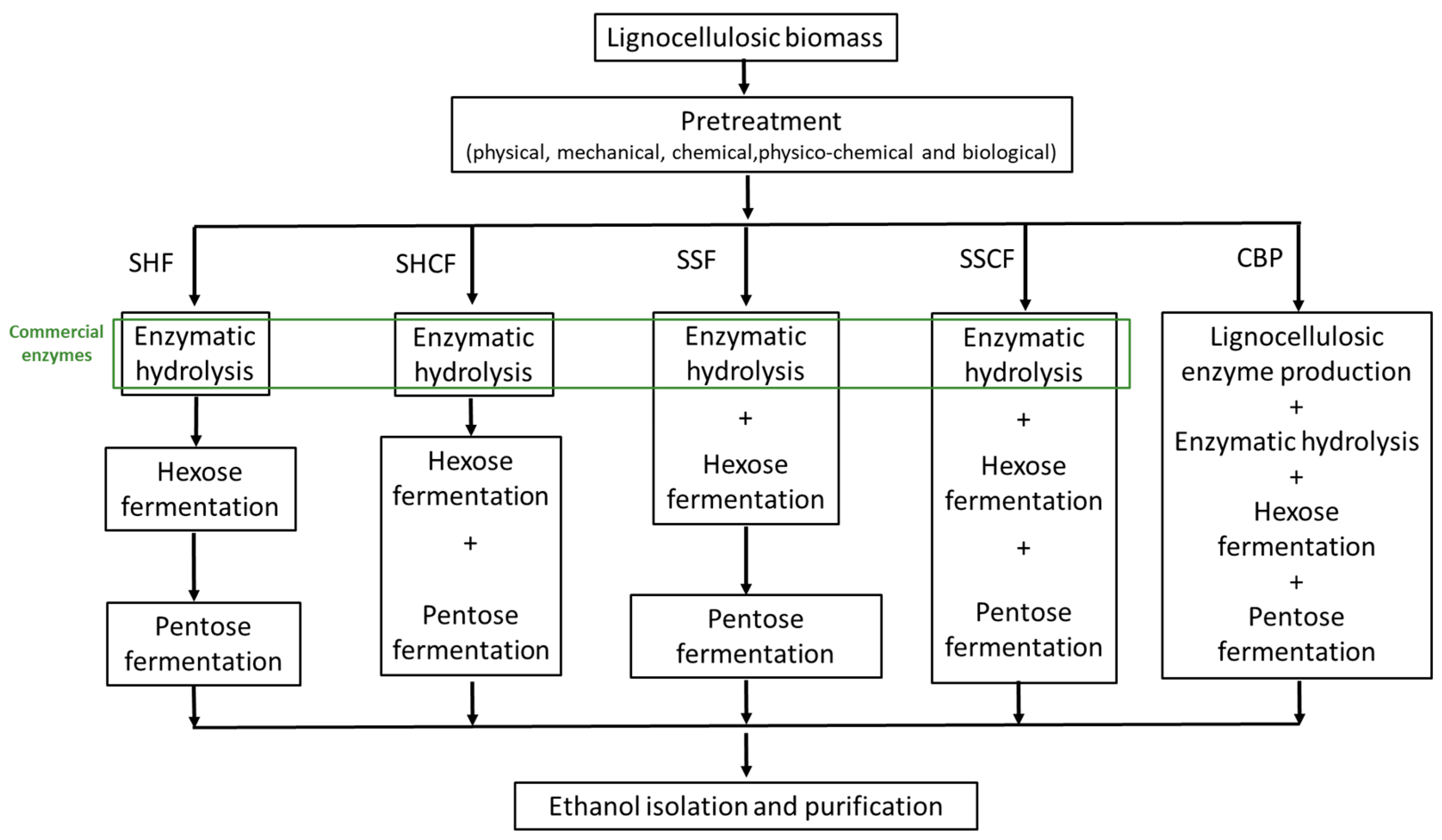
Figure 1. Process configuration of bioethanol production from lignocellulosic biomass: SHF, separate hydrolysis and fermentation; SHCF, separate hydrolysis and co-fermentation; SSF, simultaneous saccharification and fermentation; SSCF, simultaneous saccharification and co-fermentation; and CBP, consolidated bioprocess.
The biochemical route of bioethanol production involves three main steps: pretreatment, enzymatic hydrolysis, and fermentation. The pretreatment step alters the recalcitrant structure of lignocellulosic biomass, making the structural carbohydrates more accessible to hydrolytic enzymes in the subsequent step. Pretreated biomass is further hydrolyzed to simple sugars by cellulases and hemicellulases. Besides enzymes involved in the hydrolysis of carbohydrates, commercial enzymatic mixtures may also contain lignin-degrading auxiliary enzymes and lignin-modifying enzymes that improve the rate of hydrolysis and yield of simple sugars [105][13]. The reducing sugar-rich hydrolyzate obtained by enzymatic saccharification is further used as a carbon source for bioethanol production by Saccharomyces cerevisiae.
Lignocellulosic hydrolysate is mostly composed of hexoses (glucose) and pentoses (xylose and arabinose), which theoretically yield 0.51 g ethanol per 1 g of sugar. In most of the current pilot, demonstration, and commercial plants, hydrolysis of lignocellulosic biomass and fermentation of sugars are performed separately or simultaneously. Separate hydrolysis and fermentation (SHF) is a process in which hydrolysis and lignocellulosic biomass are performed in separate tanks under optimal temperature for cellulase and xylanase hydrolysis (45–50 °C) and microorganism growth (30–37 °C; Figure 1). Nevertheless, this process also has several drawbacks that outweigh these advantages, including the higher capital cost for separate tanks, cellulase inhibition by end-products (glucose and cellobiose), and microorganism inhibition by the high osmotic pressure in lignocellulosic slurry. Simultaneous saccharification and fermentation (SSF) lowers the capital costs by performing hydrolysis and fermentation in one tank, consequently decreasing the energy cost, risk of contamination, cellulase and microorganism inhibition, and process time. Furthermore, potential sugar loss is avoided by omitting the filtration step after enzymatic hydrolysis. The major drawback of this process is the conditions (i.e., pH and temperature) under which fermentation and enzyme hydrolysis are conducted. The temperature of ~37 °C at which SSFs are usually running is suboptimal for microbial growth (optimal T = 30–32 °C for yeasts) and cellulase hydrolysis (optimal T = 45–50 °C). The ethanol yield in SSF is generally higher than in SHF [106,107][14][15]. Table 1 presents several SSF and SHF processes using different lignocellulosic biomass. In order to further enhance the ethanol productivity and sugar yield by SSF, thermotolerant yeasts that are more resistant to a higher temperature range of 40–45 °C have been applied in production. Several strains, such as Kluyveromyces marxianus, Candida brassicae, and Saccharomyces uvarum have shown relatively high ethanol yields [108,109][16][17].
Lignocellulosic biomass may contain 5%–20% or more of the carbohydrates composed of pentose sugars such as xylose and arabinose [110][18]. Several techno-economic studies have shown that using pentoses for bioethanol production directly contributes to economic ethanol production [111][19]. Instead of S. cerevisiae which is unable to ferment xylose, bacterial and non-conventional yeast strains that metabolize a wide range of sugars, including xylose, can be used in bioethanol production, e.g., Candida intermedia, Scheffersomyces stipites, Candida shehatae, Kluyveromyces marxianus [108[16][20][21][22],112,113,114], and Bacillus macerans [115,116][23][24]. Despite wide substrate acceptance, the ethanol yield of these microorganisms is significantly lower than S. cerevisiae.
In order to improve the conversion yields of xylose-to-ethanol and ethanol concentration, specific traits of S. cerevisiae, non-conventional yeasts, and Escherichia coli have been modified by genetic engineering. However, the conversion of xylose to ethanol and ethanol productivity is rather low [117,118,119][25][26][27] (Table 1).
In SSH and SSF processes, cellulose- and hemicellulose-containing streams produced by pretreatment are separately fermented. Using wild-type or recombinant microorganisms capable of the simultaneous conversion of pentose and hexose to bioethanol reduces the number of steps in SSH and SSF processes regardless of whether fermentation and hydrolysis are conducted separately or simultaneously (Figure 1). Thus, bioethanol can be produced through the simultaneous scarification and co-fermentation (SSCF) of xylose and glucose or by co-fermentation of these sugars after the hydrolysis step by separate hydrolysis and co-fermentation (SHCF) [106,107][14][15].
The conversion of lignocellulosic biomass to bioethanol includes several steps depending on the process configuration, substrate composition, and microorganism characteristics, which inevitably increase the product price. Conversion of the substrate to bioethanol in a single step, used in the production of first-generation bioethanol, would reduce capital and production costs and make the process of second-generation bioethanol more economically competitive. According to techno-economical studies, the most economically feasible strategy for bioethanol production is consolidated bioprocessing (CBP) (Figure 1) [106,120][14][28]. This process combines cellulase/hemicellulase production, saccharification, and fermentation. The integrated process relies on microorganisms which, in addition to high specific growth rates, high ethanol yield, and productivity, produce enzymes for lignocellulosic biomass hydrolysis and metabolize pentose and hexose simultaneously. Wild-type microorganisms and genetically modified bacterial, yeast, and fungi strains were applied in CBP, obtaining low productivity and product yields [106,121][14][29]. An efficient and robust strain with preferred properties for industrial application has not yet been reported in the literature (Table 1). Davison et al. reported on S. cerevisiae strain coexpressing genes from Trichoderma reesei endoglucanase and Saccharomycopsis fibuligera β-glucosidase and producing 4.05 g/L ethanol with a conversion yield of 83.7% [122][30]. Another approach includes co-culturing wild-type microorganisms that produce enzymes needed for the hydrolysis of lignocellulosic biomass and the fermentation of a glucose–xylose mixture in a single step. Co-cultivation of Aspergillus oryzae and S. cerevisiae NCYC479 on brewer’s spent grain resulted in a high ethanol yield of 37 g/L [123][31]. The critical challenge in the further progress of the CBP strategy is still developing efficient microorganisms using advanced methods of genetic engineering, mutagenesis, and adaptive evolution. Alternatively, screening for microbial diversity, in particular, microbial communities living in extreme environments, might reveal novel microorganisms with a combination of favorable properties.
The low ethanol concentration in culture broth produced by batch cultivation on lignocellulosic hydrolysate negatively affects the economy of the fermentation process as well as product isolation and purification. The increased volume of the bioreactors and tanks for hydrolysis enlarges the capital costs for bioethanol production. Therefore, increasing the bioethanol concentration should be one of the goals for improving the cost-effectiveness of the process. Consequently, the bioprocess efficiency would increase, while the capital, labor, and energy costs as well as the water demand would decrease [124][32].
Thermochemical pretreatment is commonly used as a pretreatment step since it efficiently reduces the recalcitrance of lignocellulosic biomass by degrading the lignin and decreasing cellulose crystallinity. However, pretreatment generates several degradation by-products, such as furans, phenols, and organic acids, negatively affecting microorganism growth, product yield, and cellulase/hemicellulase activity. The inhibition effect is most pronounced in fermentations at high substrate loadings due to the increased inhibitor concentration [58,106,125,126][14][33][34][35]. The inhibitory effect could be decreased or avoided by applying several approaches. First, implementing SSF instead of SHF enables the microorganism to adapt to the increasing concentration of inhibitors and improve the product yield. Also, different chemical, biological, and physical methods can be applied to detoxify lignocellulosic slurries. High inoculum loading can help to overcome the inhibition effect, or instead of commonly used producing strains, one can adopt microbial species and strains exhibiting resistance to inhibitors. Finally, the adaptation of the producing strain through mutagenesis or improvement through genetic engineering can also be applied [126][35].
A critical factor in developing a cost-effective biorefinery includes utilizing low-valued feedstocks such as lignocellulosic biomass and efficient conversion into biofuels and different bio-based products that generate income and contribute to the economic feasibility of production. Regardless of the pretreatment method and fermentation configuration employed in bioethanol production, lignin-rich waste streams in the form of black liquor after the pretreatment step and solid (together with cell biomass) obtained by centrifugation of the culture broth after fermentation are obtained. Depending on the lignocellulosic biomass composition and production process, these streams can contain more than 20% (w/w) of lignin calculated based on the total lignocellulosic biomass weight used in the process. Lignin can be used as an energy source for power generation in biorefineries and feedstock for the production of biofuels and valuable chemicals (such as asphalts, bioplastics, biopolymers, and resins). Furthermore, the sugar streams generated in the process can also be used to develop several sugar-based coproducts (Figure 1, [127][36]).
Table 1. The efficiency of bioethanol production related to the pretreatment method, used microorganisms, and process configuration.
| Lignocellulosic Biomass | Pretreatment | Enzyme Hydrolysis/Substrate and Conditions | Microorganism | Process Config. |
Major Findings | Ref. |
|---|---|---|---|---|---|---|
| Oil palm empty fruit bunches | Two-step pretreatment: (1) 0.2 M H2SO4 at 121 °C for 53 min, and (2) 0.2 M H2SO4 at 121 °C for 53 min; biomass loading: 12.50% (w/v) | Enzyme: 20 FPU cellulase/gLCB a and 4 IU ß-glucosidase/gLCB b substrate loading 10% (w/v) hydrolysis at 37.5 °C for 72 h; |
K. marxianus | SHF, batch | Ethanol yield: 0.258 g/gLCB c Ethanol concentration 25.80 g/L |
[128][37] |
| As above | As above | Enzyme: 20 FPU cellulase/gLCB a and 4 IU ß-glucosidase/gLCB b | As above | SSF, batch | Ethanol yield 0.281 g/gLCB c Ethanol concentration 28.10 g/L Substrate loading: 10% (w/v) |
[128][37] |
| g Woody and herbaceous biomass | h Steam explosion conditions: 190–210 °C, 2–8 min depending on lignocellulosic biomass |
Enzyme: 15 FPU/gLCB a | K. marxianus CECT10875 | SSF, batch | Ethanol concentration 16.2–19.0 g/L Ethanol yield 60.9–71.2% of theoretical yield Fermentation temperature: 42 °C |
[129][38] |
| Corncob residue | KOH pretreatment | Enzyme: 22 FPU cellulase/gLCB a substrate loading 7.5% (w/v) |
S. cerevisiae TC-5 | SSF, fed-batch | Ethanol concentration 31.96 g/L Ethanol productivity 0.222 g/L h Fermentation temperature: 40 °C |
[130][39] |
| Wheat straw | Steam explosion: 220 °C and 2.5 min | Enzyme: 15 FPU cellulase/gC e and 15 IU ß-glucosidase/gC f | Kluyveromyces marxianus CECT 10875 | SSF, fed-batch | Ethanol concentration 36.2 g/L Ethanol yield: 0.33 g/gG d Substrate loading: initial 10 (w/v) + 4% (w/v) addition after 12 h; Fermentation temperature: 42 °C |
[131][40] |
| Sugarcane bagasse | Steam pretreatment with 0.5% (w/v) H2SO4 at 121 °C for 30 min. | 15 FPU/ gLCB a | Saccharomyces cerevisiae | SSF, fed-batch | Ethanol concentration 65.43 g/L Cumulative substrate concentration ~20% (w/w) |
|
| Corn cobs | 2% NaOH at 120 °C for 15 min; solid-to-liquid ratio of 1:5 (w/v) | - | S. cerevisiae YI13 co-producing BGLI and EGII | CBP, batch | Ethanol concentration 4.05 g/L Conversion yield (83.7%) after 168 h |
[122][30] |
| Brewers spent grains | Dried and ground | - | Co-culture Aspergillus oryzae and S. cerevisiae NCYC479 | CBP, batch | Ethanol concentration 37 g/L after 10-day incubation at 15 °C | [123][31] |
a Cellulase loading, FPU per g of lignocellulosic biomass (FPU/gLCB); b ß-glucosidase loading, IU per g of lignocellulosic biomass (U/gLCB); c ethanol yield, g of ethanol per g of lignocellulosic biomass (g/gLCB); d ethanol yield, g of ethanol per g of total glucose in the pretreated lignocellulosic biomass (g/gG); e cellulase loading, FPU per g of cellulose in lignocellulosic biomass (FPU/gC); f ß-glucodidase loading, IU per g of cellulose in lignocellulosic biomass (U/gC); g lignocellulosic woody (poplar and eucalyptus) and herbaceous (Sorghum sp. bagasse, wheat straw and Brassica carinata residue) biomass; hpoplar and eucalyptus biomass, 210 °C, 4 min; wheat straw, 190 °C, 8 min; sweet sorghum bagasse, 210 °C, 2 min and B. carinata residue, 210 °C, 8 min.
4. Bioethanol Recovery and Purification
The final step of bioethanol production is the recovery of the product from the culture broth, commonly achieved by conventional distillation. Dehydration methods are further applied to reduce the water content below 0.2% (v/v) or 1.0% (v/v) according to two well-established fuel standards, EN 15376 (Europe) and ASTM D 4806 (USA), respectively [132,133][41][42]. Distillation and dehydration are the most energy-intensive steps significantly contributing to greenhouse gas emissions (e.g., CO2). A low bioethanol concentration in the culture broth adds significant capital and operating costs and negatively affects the energy balance of the process. The concentration of bioethanol produced by different bioprocesses using sugar-based substrates is typically between 5–12% (vol /vol), while the concentration of bioethanol produced from lignocellulosic biomass is lower, below 40 g/L (Table 1). According to several studies, the distillation of culture broth with an ethanol concentration below 40 g/L is economically unfeasible [124,134][32][43]. Distillation is the most common unit operation based on the relative volatility or boiling temperature difference of the components in the mixture. Distillation of the water–ethanol mixture results in an azeotrope binary mixture containing 95.6% (wt) ethanol at 78.15 °C, and further enrichment of the vaporous phase with ethanol is not possible. Therefore, the process of ethanol recovery and purification on a large scale is conducted in two steps. Diluted ethanol solution, i.e., the culture broth is first concentrated by ordinary distillation at atmospheric pressure to 92.4% (wt) ethanol, followed by dehydration. Several separation methods have been developed for recovering anhydrous ethanol (>99.5%, wt) which include various distillation techniques and hybrid processes that combine distillation with other unit operations for breaking the azeotrope, like adsorption (distillation + molecular sieves) or pervaporation (distillation + membranes) [135,136][44][45].
4.1. Distillation-Based Processes
In order to separate ethanol from the azeotropic mixture by distillation, the condition and configuration of the distillation process have been modified. Several distillation techniques used for breaking the azeotropic mixture have been reported in the literature: simple vacuum distillation, azeotropic distillation, extractive distillation, and pressure-swing distillation. However, these distillation techniques are tremendously energy-demanding and require high capital costs. Anhydrous ethanol can be obtained by simple distillation under low pressure (0.11 atm), i.e., vacuum distillation. However, due to high operational costs, vacuum distillation is not applied in industrial ethanol separation [135][44].
In azeotropic distillation, the third component, the entrainer, is added to the azeotropic ethanol–water mixture in order to modify the equilibrium and obtain pure products. The entrainer forms a ternary azeotropic mixture, changing the relative volatilities of the other two components. Various entrainers for the separation of ethanol–water mixtures have been studied: benzene [136,137[45][46][47],138], toluene [139][48], cyclohexane [136][45], gasoline additives (tert-amyl methyl ether, TAME) [140][49], etc. Azeotropic distillation is conducted using two columns: first, a dehydration column for the generation of pure ethanol (>99%), and second, an entrainer column for entrainer recovery. The water–ethanol mixture is introduced into the dehydration column (first column), and the entrainer is fed above the feed tray. The entrainer is recovered from the overhead area of the column, while bioethanol is collected at the bottom. The entrainer can form a homogenous (homogenous azeotropic distillation) or heterogeneous mixture (heterogeneous azeotropic distillation) with ethanol–water solution along the distillation column. If a heterogeneous mixture is formed, the overhead vapor is condensed and fed to decenter to separate the ethanol–entrainer and water–entrainer streams. The ethanol–entrained phase is refluxed back to the dehydration column (first column), while the water–entrainer phase is further processed in the second column for the recovery of the entrainer which is fed to the dehydration column [135][44].
Like azeotropic distillation, extractive distillation also requires an entrainer to separate ethanol from an aqueous solution. For a long time, it was considered as a special type of azeotropic distillation. However, these two types of distillations have different process configurations and obey different feasibility rules [141][50]. Various types of entrainers have been studied in the literature: liquid solvents (e.g., ethylene glycol [142][51], glycerol [143][52], dissolved salts (e.g., lithium chloride, calcium chloride, sodium chloride, and potassium chloride [144][53]), ionic liquids (e.g., 1-butyl-3-methylimidazolium chloride, 1-ethyl-3-methylimidazolium tetrafluoroborate) [145][54], 1-ethyl-3-methylpyridinium ethylsulfate [146][55], hyperbranched polymers (polyesters and polyesteramides and polyethylene glycol) [147][56], and complex solvents [148][57]. Extractive distillation configuration depends on the entrainer used for ethanol purification. Conventionally, liquid solvent with a high-boiling point used as an entrainer is introduced in the extractive distillation column above the ethanol–water feed. The extractive column is divided into three sections: the rectifying section (above the entrainer feed), the extractive section (between two feeds), and the stripping section (stages below the ethanol–water mixture feed). The addition of an entrainer increases the relative volatility of the ethanol without forming the new azeotrope. Ethanol goes to the top of the column while water collects at the bottom of the distillation column with added solvent. The solvent is recovered by distillation and returned to the extractive section of the extractive column [149][58]. The main disadvantage of this type of extractive distillation is the high solvent-to-feed (ethanol–water) mass ratio, which exceeds 5:1, entailing large energy consumption for costs involved in reboiler and condenser duty (extractive distillation) and entrainer recovery (second distillation column). A substantial energy saving could be achieved using soluble salts instead of liquid solvents. Dissolving salt in an ethanol–water mixture enhances the relative volatility of ethanol due to the salting-out effect on ethanol. The salt is directly introduced at the top or near the top trey of the extractive distillation column. Salt dissolves in the reflux stream, flows downward along the column concurrent with the water stream, and collects as the bottom product. It is further recycled by evaporation or drying. Ethanol (extracted component) evaporates due to higher relative volatility compared to water and collects as a top distillate product free of the entrainer. When potassium acetate is used as an entrainer instead of ethylene glycol, the ratio of entrainer to feed is reduced by 50 times (0.06 mol/mol). Due to the low entrainer-to-feed ratio, extraction distillation with dissolved salt is much less energy-intensive and has a higher production capacity than extraction distillation with liquid solvents. Furthermore, salts are less toxic and not volatile, so they do not contaminate the overhead product. Ionic liquids (ILs) have also been investigated as alternative entrainers that could replace conventional volatile organic solvents in extractive distillation due to unique properties such as non-volatility, thermal stability, environmental benignity, and easy recovery after extraction. The addition of ILs has a similar effect on the separation of components of an azeotropic mixture such as dissolved salt. ILs can be easily recovered using simple flash distillation or gas stripping [146,150][55][59].
Unlike the other distillation methods, pressure-swing distillation does not depend on an entrainer to separate the ethanol–water azeotrope. It relies on the change in azeotrope composition with applied pressure. Two distillation columns are used, the first operating at high pressure and the second operating at low pressure. In the case of an azeotrope with minimum boiling, such as water–ethanol, high-purity product streams are collected on the bottom of columns while the distillate stream from the high-pressure column is recycled back to the low-pressure column. Iqbal and Akhlaq produced nearly pure ethanol (99.7%, mol/mol) obtained at the bottom of the high-pressure column (10 atm), while water (99.5%, mol/mol) was collected at the bottom of the first column (1 atm) [151][60].
42. Adsorption
4.2. Adsorption
Adsorption is a cost-effective and environmentally friendly alternative to extractive and azeotrope distillation and is used on a large scale to purify ethanol. Many traditional porous adsorbents have been applied to capture either water or ethanol from distillates and culture broths, such as zeolites [152[61][62][63][64],153,154,155], silicate [156][65], activated carbons [157][66], composite adsorbents (e.g., polyvinyl alcohol/zeolite/carbon composites, and composite silica–divinylbenzene) [158[67][68][69],159,160], polymeric resins, activated carbons metal−organic frameworks [160[69][70],161], etc. Biomass has also been investigated as an alternative adsorbent since it is an abundant, eco-friendly, inexpensive, and easily regenerated material. Various starch and lignocellulose-based biomaterials have been applied for that purpose, including canola meal [162][71], oat hull, flax shives [163][72], cassava starch [164][73], rice straw, and paddy husk [165][74].
Depending on the configuration of the ethanol production and purification process, the adsorbent is applied to the ethanol–water mixture in the vapor or liquid phase. A vaporous stream leaving the top of the distillation column after the first purification step by conventional distillation can be directly introduced to the adsorption column, avoiding additional vaporization steps and reducing production costs. Regeneration of the adsorbent for next cycle application is usually carried out by heating (for zeolites: 200–250 °C), lowering the pressure, and reducing the pressure in combination with purging the adsorbent bed with an inert gas [152][61].
The similar physical–chemical properties of water and ethanol make the search for suitable adsorbents challenging. Both molecules are highly polar and bind strongly on common adsorbents. The molecular size and polarity of water and ethanol are 2.8 and 4.4 A and 0.36 and 0.65, respectively [135,152,153][44][61][62]. The separation efficiency depends on the pore dimensions of the microporous structure of the adsorbent with respect to the molecular sizes or interactions between the adsorbent and components of the mixture. The adsorbent can also be applied to the liquid phase containing ethanol–water binary mixtures or complex mixtures such as fermentation broth. The 3A and 4A zeolite molecular sieves (with microspores smaller than 3 and 4 Angstroms, respectively) have been used to dry ethanol from liquid and vapor ethanol–water mixtures. Unlike ethanol, water molecules easily penetrate into the microporous channels of molecular sieves and adsorb [152,153][61][62].
Although bio-based adsorbents have lower separation capacity than adsorbents applied to large-scale ethanol purification (e.g., zeolites), the main advantage of using adsorbents is the possibility of omitting the regeneration step. The water-saturated adsorbent can be directly used as feedstock (substrate) for bioethanol production and fresh biomass for the ethanol dehydration step. Many conventional adsorbents have been studied for ethanol separation, but most of them exhibit stronger interactions with water than ethanol. Since water is the main component in the cultivation broth, it is essential to capture the component present in relatively low concentrations, i.e., ethanol. Therefore, designing new adsorbents that selectively capture ethanol from dilute ethanol mixtures is crucial for the feasibility of bioethanol production. Another advantage of in-situ ethanol removal is an increase in process productivity. Applying zeolite NaZSM-5 for in situ ethanol recovery during fermentation in bioreactor growth inhibition by-products was avoided, improving the process efficiency [166][75].
4.3. Membrane Separation
Over the last few decades, membrane separation processes and materials have been extensively studied and evaluated. The membrane process emerged as a sustainable and energy-efficient alternative to conventional azeotropic distillation. Membrane systems are built in a modular form that is adaptive to any particular demand. The main characteristics are easy operation, low maintenance, and low space requirements. Different membrane processes have been employed for bioethanol isolation and dehydration, including membrane distillation [167][76], pervaporation [168[77][78][79],169,170], vapor permeation [171][80], nanofiltration [172][81], reverse osmosis [173][82], and forward osmosis [174][83].
Pervaporation is one of the most studied processes for ethanol dehydration (ethanol–water mixture with high ethanol concentration) and isolation and purification from diluted mixtures, i.e., culture broth. The membranes comprise a selective layer that accomplishes separation and a porous support layer to provide mechanical strength. The ethanol-containing stream is brought into contact with the selective side of the membrane. The membrane acts as a semipermeable barrier that allows specific component(s) to permeate the membrane to the permeate side and retain the other components of a mixture (retenate). The three steps in pervaporation separation are the sorption of the specific component on the feed side of the membrane, permeation through the membrane, and desorption by evaporation on the permeate side where the permeate vapor is condensed at atmospheric pressure (condenser). The driving force for molecules’ permeation through the pervaporation membrane is a difference in chemical potential on both sides of the membrane. The chemical potential difference is maintained by purging with inert gas (sweep gas pervaporation) or applying a vacuum (vacuum pervaporation) on the permeate side of the membrane or by keeping the temperature difference (thermopervaporation) on both sides of the membrane [135,175][44][84].
Pervaporation membranes are symmetric (dense) and asymmetric (dense/porous) based on their morphology. Due to limited permeate flux through relatively thick layers, dense membranes are unsuitable on an industrial scale. Asymmetric membranes composed of a thin separation layer on the surface of microporous support has a higher total flux of permeate compared to dense membranes. Pervaporation membranes are manufactured in three types of geometries: flat-sheet, tubular, and hollow-fiber membranes [168][77]. Membranes used for the separation of binary ethanol–water mixtures are ethanol-selective (hydrophobic) or water–selective (hydrophilic). Pervaporation with ethanol-selective hydrophobic membranes is considered the most promising alternative to the conventional processes currently used for ethanol recovery on a large scale since the concentration of ethanol in the culture broth is rather low. Based on the materials used for manufacturing membranes, they are polymeric, inorganic, and mixed-matrix membranes made of polymeric and inorganic materials [135,175,176][44][84][85].
Inorganic membranes are produced from silica, alumina (Al2O3), or zeolite. Due to their narrow pore size distribution and intrinsic surface properties, these membranes exhibit excellent separation performance, including having a high separation factor and permeation flux. Furthermore, inorganic membranes have high solvent resistance, temperature stability, and mechanical strength. Unlike polymeric membranes, they do not suffer from swelling problems [177][86]. Zeolites are hydrated aluminosilicates characterized by pores and cavities of molecular dimensions. Zeolite membranes consist of a polycrystalline zeolite layer on top of a porous inorganic layer which provides mechanical strength with low mass-transfer resistance. These membranes can be water- or ethanol-selective. The most studied hydrophobic ethanol selective zeolite membranes are silicate-1 (pure silica) and ZSM-5 (some Si atoms substituted by Al). The silicate-1 membrane is more hydrophobic than ZSM-5 since it does not contain aluminium atoms in its structure. Incorporating tri and tetravalent elements, such as aluminium, boron, germanium, and iron zirconium, into a zeolite structure changes the pore size and hydrophobicity of the membrane, affecting the sorption and diffusion properties of the zeolite material and changing the membrane flux and selectivity (e.g., Al-ZSM-5, B-ZSM-5, etc.) [168][77]. The best-performing ethanol selective membrane, Ti-silicate-1, with a separation factor of 127 and permeate flux of 770 g m−2 h−1 has substituted titanium atoms into the silicate-1 structure [Chen et al., 2008]. For the dehydration of ethanol solutions containing 5–10% of water the following inorganic materials are used: hybrid silicas and three types of zeolite, Linde Type A (NaA), Chabazite (CHA) and T-type, and are reported in the literature. The Linde Type A (LTA) zeolite has incorporated sodium counter ions in the 1:1 Si:Al aluminosilicate lattice, also known as NaA or 4A zeolite [169,178][78][87]. The stability of NaA membranes in acidic and high-water activity environments has been improved using more stable zeolite materials recently developed and commercialized. Lower Al content in T-type membranes compared to NaA zeolite decreases their hydrophobicity and improves acid stability [169][78].
Mixed-matrix membranes (MMM) combine the favorable characteristics of inorganic and polymeric membranes, easy processability and low costs of polymeric materials, and the superior selectivity and high stability of inorganic materials. They consist of inorganic moieties in the form of micro or nano-particles (discrete phase) incorporated into a polymer matrix (continuous phase) [168,170][77][79]. Most of the ethanol-selective mixed-matrix membranes are based on polydimethylsiloxane (PDMS), although other materials have been studied, such as poly(1-trimethylsilyl-1-propyne); PTMSP [179[88][89],180], poly(ether block amide) [181][90], poly(methylphenylsiloxane) [182][91], etc. The most common inorganic materials used as filler are zeolites (silicalite-1 and ZSM-5) [183][92], fumed silica [184][93], metal–organic frameworks [185][94], zeolitic imidazolate frameworks (ZIFs) [186][95], and carbon nanotubes [187][96], etc.
Diverse polymeric materials have been extensively investigated as potential materials for pervaporation membrane manufacturing. Hydrophobic polymeric membranes made of polydimethylsiloxane (PDMS) and poly(1-trimethylsilyl1-propyne) (PTMSP) have been widely applied in ethanol removal from diluted ethanol solution. The separation factor and permeation fluxes for the PDMS membrane are generally below 10 and 1000 g m−2 h−1, respectively. PTMSP membranes exhibit better separation characteristics than PDMS membranes (so-called silicone rubber), with separation factors ranging from 9 to 26. Despite good performance characteristics, PTMSP membranes have not been applied on a large scale due to membrane deterioration caused by the chemical and physical degradation of polymer structure. In order to improve the separation characteristics and stability, PDMS and PTMSP membranes have been modified. Various modifications of these membranes have been reported in the literature, including blending, blocking, or grafting with different polymers by incorporating fillers forming the mixed-matrix membrane (MMM) [168][77]. Furthermore, asymmetric composite membranes consisting of thin selective layers of polymer on highly porous support material exhibited improved separation performance. Li et al. synthesized a composite membrane consisting of a PDMS ultra-thin selective layer (thickness from 0.5 to 8 μm) on porous polytetrafluoroethylene (PTFE) support. The membrane exhibits a high flux of 2016 g m−2 h−1 with a separation factor of 12 for the separation of ethanol from a 5 wt% solution with stable operation over 200 h [188][97]. Furthermore, several other polymers have been proposed for the recovery of alcohol diluted aqueous solutions by pervaporation, including homopolymers and copolymers of siloxane (e.g., polymethylethoxysiloxane (PMES), polymethylphenylsiloxane (PMPS), polyphenylmethylsiloxane (PPMS), polydimethylsiloxane-imide (PDSI), polysiloxaneimides (PSI), polyoctylmethylsiloxane (POMS), etc.), poly(vinyltriethoxysilane) (PVTES) and PVTES copolymerized with other substances (e.g., dimethyldiethoxysilane and PDMS), poly(ether block amide) (PEBA), polytetrafluoroethylene (PTFE), perfluoroprpane (PFP), etc. [168][77]. Compared to inorganic membranes, polymeric membranes have high processability, lower separation performance (separation factor, permeate flux, and stability), and low cost [189][98].
44. Advanced Hybrid Processes
4.4. Advanced Hybrid Processes
The high operational costs of the distillation-based process encourage the development of new innovative separation processes. Integrating bioethanol separation with the fermentation process enables continuous removal to maintain bioethanol at low concentrations. Avoiding yeast inhibition increases ethanol yield and process efficiency, shortens the fermentation time, and decreases the production costs. Several advanced hybrid processes for in situ bioethanol removal have been described in the literature, which combine fermentation with vacuum fermentation [190[99][100],191], flash fermentation [113,191[21][100][101],192], gas stripping [193[102][103],194], adsorption [195[104][105][106],196,197], solvent extraction [198[107][108][109][110][111],199,200,201,202], and membrane pervaporation [168,203,204][77][112][113].
In vacuum fermentation, a bioreactor is maintained under vacuum using a vacuum pump, allowing ethanol to boil off from the culture broth at the fermentation temperature. Ethanol is recovered by condensation in the condenser. Also, carbon dioxide, a known inhibitor of yeast metabolism, is also removed, improving the fermentation rate. Due to continuous product and by-product removal, high productivity is obtained, especially when substrate feeding is applied (fed-batch cultivation). Huang et al. studied bioethanol production by vacuum fermentation using food waste as substrate at high solid loading (35%, w/w) and obtained complete sugar conversion and increased the product yield (358 g of ethanol /kg substrate by vacuum fermentation versus 327 g of ethanol /kg substrate in conventional fermentation) [190][99]. However, several difficulties are connected with applying a vacuum during fermentation. Carbon dioxide generated by yeast metabolism must be compressed from the bioreactor pressure up to atmospheric pressure, increasing production costs [205][114]. Furthermore, yeast cells have to be supplied with oxygen to ensure growth and product accumulation. Instead of air, culture broth has to be aerated by pure oxygen due to the low solubility of oxygen under reduced pressure [191][100].
These difficulties are overcome in flash fermentation, where the fermentation process is separated from ethanol recovery. The low ethanol concentration is maintained by the periodical circulation of culture broth to a vacuum chamber while the bioreactor remains at atmospheric pressure. Since the fermentation is conducted at atmospheric pressure, dissolved oxygen levels can be maintained by adding air instead of pure oxygen. Furthermore, this process configuration enables a reduction in compression costs since carbon dioxide is not compressed [191,192][100][101].
Gas stripping is a simple, low-cost method for the in situ removal of ethanol. Stripping gas, air, CO2, or N2 is sparged in the culture broth, causing the evaporation of volatile compounds, including bioethanol, which is further recovered in a condenser. The main benefits of in situ ethanol removal are the improvement of productivity and the reduction in process water and energy use, especially in fermentation conducted at higher substrate loadings. Nevertheless, applying high substrate loadings in batch and fed-batch fermentations accumulates toxic non-volatile compounds in culture broth that can originate from yeast metabolism or pretreated lignocellulosic biomass [194][103]. Wang et al. continuously removed produced bioethanol during the simultaneous saccharification and solid-state fermentation of rice straw using nitrogen as the stripping gas. In situ product removal resulted in low concentrations of bioethanol and high substrate consumption [193][102].
Bioethanol and inhibitors from pretreated lignocellulosic biomass can be removed by liquid–liquid extraction by mixing culture broth with organic solvent during fermentation. Desirable solvent characteristics are high capacity for ethanol (described by equilibrium distribution coefficient, KD), high selectivity for ethanol over water, and biocompatibility (nontoxicity) with producing microorganisms. The extracted bioethanol can be recovered by distillation or back extraction. Numerous solvents were tested as extractants for ethanol recovery from culture broth, including alcohols, esters, alkanes, hydrocarbons, synthetic aliphatic hydrocarbon solvents, ketones, amines, acids, chlorinated hydrocarbons, aromatics, polymers, ionic liquids, etc. [198,199,200,201][107][108][109][110]. Despite high selectivity, alcohols and esters show toxicity to ethanol-producing microorganisms [198][107]. In order to avoid cell death, extraction can be conducted in separate containers with cell-free culture broth instead of in a bioreactor. The test against the producing microorganism showed that toxicity of alcohol decreases with carbon chain length. Thus, toxicity experiments showed growth inhibition of Zymomonas mobilis by n-amyl alcohol. However, using n-amyl alcohol as a solvent in extractive fermentation with Zymomonas mobilis improved ethanol yield and productivity compared to conventional fermentation [202][111].
Adsorption by a molecular sieve is a commonly used method for removing the remaining water from bioethanol–water mixtures in the final step of the commercial production of fuel-grade bioethanol. The adsorption method has also been applied in several studies for the in situ removal of bioethanol from culture broth during fermentation to maintain the low bioethanol concentration and avoid inhibition. The main disadvantages of the direct application of adsorbent in fermentation broth are a decrease in adsorbent capacity due to the unspecific binding of microbial cells and fermentation broth components (nutrients and cell metabolites), the toxicity of adsorbents to microbial cells, a decrease in adsorbent lifetime due to irreversible adsorption, etc. The toxicity of the adsorbents to cells could be overcome by using immobilized microorganisms instead of free cells or by conducting the adsorption in separate steps (e.g., fluidized bed or fixed-bed column) with cell-free culture broth after cell separation [191,195,196,197][100][104][105][106]. Jones et al. used F-600 activated carbon in bioethanol fermentation using E. coli KO11 (ATCC 55124). The direct addition of an optimized quantity of adsorbent to the fermentation broth improved ethanol yield and resulted in the complete depletion of the carbon source [195][104]. Hashi et al. studied in situ bioethanol removal by combining carbon dioxide stripping and the adsorption of bioethanol onto activated carbon WV-B 1500. A mathematical model was developed to predict the performance of the adsorption column [196][105]. A similar approach was applied by Seo et al. in recovering pure bioethanol. Bioethanol was first recovered from fermentation broth in three steps: gas stripping of bioethanol from the culture broth, preconcentration from the gaseous phase by adsorption onto molecular-sieving carbon (5A), and selective dehydration driven by the molecular-sieving effect during the desorption phase [197][106].
Pervaporation is an effective and energy-efficient separation method for the dehydration of alcohols from water–alcohol mixtures like ethanol, isopropanol, butanol, etc. Nowadays, pervaporation is commercially used for the dehydration of the azeotropic ethanol-water mixture at a large scale in the production of fuel-grade bioethanol. Pervaporation is also a promising method for in situ bioethanol recovery from diluted complex mixtures such as fermentation broth since the pervaporation membranes are nontoxic to microorganisms and conditions under which the pervaporation process is conducted, which do not influence cell growth [206][115]. Polydimethylsiloxane (PDMS) is mostly studied for the separation of ethanol from aqueous solution on a lab scale due to its excellent stability and satisfactory separation performance [203][112]. The dramatic decline in pervaporation membrane performance (bioethanol permeability and selectivity) is observed upon membrane exposure to fermentation broths. Deterioration of membrane performance is attributed to the adhesion of components in culture broth such as sugars, inorganic salts, extracellular polymers like lipids, polysaccharides and peptides, cell metabolites, and biofouling of the membrane surface through the adhesion of microorganisms and growth in the form of biofilm [168,203][77][112]. Therefore, the design of a hydrophobic pervaporation membrane with anti-fouling properties is critical for the application of pervaporation in the recovery of bioethanol from fermentation broth. Lowering the membrane surface hydrophobicity not only decreases the adhesion of culture broth components and cells to a membrane but also decreases separation performance. Biofouling of the membrane could be decreased by using immobilized cells in a fluidized bed or fixed-bed column instead of free cells (stirred tank bioreactor) or cell-free culture broth (after cell harvesting by centrifugation or microfiltration), which can be used as a feed stream in the pervaporation module [204][113]. Vapor permeation, currently used in the dehydration of organic solvents from aqueous mixtures at an industrial scale, is a more suitable method for the separation of bioethanol from fermentation broth since direct contact between the membrane and the culture broth is avoided [207,208][116][117].
References
- Khuong, L.S.; Masjuki, H.H.; Zulkifli, N.W.M.; Mohamad, E.N.; Kalam, M.A.; Alabdulkarem, A.; Jamshaid, M. Effect of gasoline–bioethanol blends on the properties and lubrication characteristics of commercial engine oil. RSC Adv. 2017, 7, 15005–15019.
- IEA. Biofuel Production by Country/Region and Fuel Type, 2016–2022; IEA: Paris, France, 2021; Available online: https://www.iea.org/data-and-statistics/charts/biofuel-production-by-country-region-and-fuel-type-2016-2022 (accessed on 9 January 2023).
- U.S. Department of Energy. Energy Efficiency and Renewable Energy. Available online: https://afdc.energy.gov/fuels/ethanol_blends.html (accessed on 9 January 2023).
- Maity, S.K. Opportunities, recent trends and challenges of integrated biorefinery: Part I. Renew. Sustain. Energy Rev. 2015, 43, 1427–1445.
- de Jong, E.; Jungmeier, G. Biorefinery concepts in comparison to petrochemical refineries. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33.
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels–a review. Int. J. Energy Res. 2020, 44, 9266–9282.
- Grubišić, M.; Šantek, B.; Zorić, Z.; Čošić, Z.; Vrana, I.; Gašparović, B.; Čož-Rakovac, R.; Šantek, M.I. Bioprospecting of Microalgae Isolated from the Adriatic Sea: Characterisation of Biomass, Pigment, Lipid, Fatty Acid Composition, Antioxidant and Antimicrobial Activity. Molecules 2022, 27, 1248.
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuel. Bioprod. Biorefin. 2019, 13, 994–1014.
- Hoekman, S.K. Biofuels in the US–challenges and opportunities. Renew. Energ. 2009, 34, 14–22.
- Akbarian, A.; Andooz, A.; Kowsari, E.; Ramakrishna, S.; Asgari, S.; Cheshmeh, Z.A. Challenges and opportunities of lignocellulosic biomass gasification in the path of circular bioeconomy. Bioresour. Technol. 2022, 362, 127774.
- Pecha, M.B.; Arbelaez, J.I.M.; Garcia-Perez, M.; Chejne, F.; Ciesielski, P.N. Progress in understanding the four dominant intra-particle phenomena of lignocellulose pyrolysis: Chemical reactions, heat transfer, mass transfer, and phase change. Green Chem. 2019, 21, 2868–2898.
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392.
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.H.; Dong, C.D. Lignin valorisation via enzymes: A sustainable approach. Fuel 2022, 311, 122608.
- Salehi Jouzani, G.; Taherzadeh, M.J. Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: A comprehensive review. Biofuel Res. J. 2015, 2, 152–195.
- Santek, M.I.; Beluhan, S.; Santek, B. Production of microbial lipids from lignocellulosic biomass. In Advances in Biofuels and Bioenergy; Nageswara-Rao, M., Soneji, J.R., Eds.; IntechOpen: London, UK, 2018; pp. 137–164.
- Leonel, L.V.; Arruda, P.V.; Chandel, A.K.; Felipe, M.G.A.; Sene, L. Kluyveromyces marxianus: A potential biocatalyst of renewable chemicals and lignocellulosic ethanol production. Crit. Rev. Biotechnol. 2021, 41, 1131–1152.
- Spindler, D.D.; Wyman, C.E.; Mohagheghi, A.; Grohmann, K. Thermotolerant yeast for simultaneous saccharification and fermentation of cellulose to ethanol. Appl. Biochem. Biotechnol. 1988, 17, 279–293.
- Hahn-Hägerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Lidén, G.; Zacchi, G. Bio-ethanol–the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556.
- Carpio, R.R.; Secchi, S.G.; Barros, R.O.; Oliveira, R.A.; Queiroz, S.; Teixeira, R.S.S.; Secchi, A.R. Techno-economic evaluation of second-generation ethanol from sugarcane bagasse: Commercial versus on-site produced enzymes and use of the xylose liquor. J. Clean. Prod. 2022, 369, 133340.
- Moreno, A.D.; Tomás-Pejó, E.; Olsson, L.; Geijer, C. Candida intermedia CBS 141442: A novel glucose/xylose co-fermenting isolate for lignocellulosic bioethanol production. Energies 2020, 13, 5363.
- Farias, D.; Atala, D.I.P.; Maugeri, F. Improving bioethanol production by Scheffersomyces stipitis using retentostat extractive fermentation at high xylose concentration. Biochem. Eng. J. 2017, 121, 171–180.
- Guan, D.; Li, Y.; Shiroma, R.; Ike, M.; Tokuyasu, K. Sequential incubation of Candida shehatae and ethanol-tolerant yeast cells for efficient ethanol production from a mixture of glucose, xylose and cellobiose. Bioresour. Technol. 2013, 132, 419–422.
- Schepers, H.-J.; Bringer-Meyer, S.; and Sahm, H. Fermentation of D-xylose to ethanol by Bacillus macerans. Z. Für Naturforschung 1987, 42, 401–407.
- Dogaris, I.; Mamma, D.; Kekos, D. Biotechnological production of ethanol from renewable resources by Neurospora crassa: An alternative to conventional yeast fermentations? Appl. Microbiol. Biotechnol. 2013, 97, 1457–1473.
- He, B.; Hao, B.; Yu, H.; Tu, F.; Wei, X.; Xiong, K.; Xia, T. Double integrating XYL2 into engineered Saccharomyces cerevisiae strains for consistently enhanced bioethanol production by effective xylose and hexose co-consumption of steam-exploded lignocellulose in bioenergy crops. Renew. Energ. 2022, 186, 341–349.
- Valdehuesa, K.N.G.; Ramos, K.R.M.; Nisola, G.M.; Bañares, A.B.; Cabulong, R.B.; Lee, W.-K.; Liu, H.; Chung, W.-J. Everyone loves an underdog: Metabolic engineering of the xylose oxidative pathway in recombinant microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 7703–7716.
- Chacón, A.; Martinez, A.; Poggi-Varaldo, H.M.; Villa-Tanaca, L.; Ramos-Valdivia, A.C.; Ponce-Noyola, T. Xylose Metabolism in Bioethanol Production: Saccharomyces cerevisiae vs. Non-Saccharomyces Yeasts. BioEnergy Res. 2021, 15, 905–923.
- Aui, A.; Wang, Y.; Mba-Wright, M. Evaluating the economic feasibility of cellulosic ethanol: A meta-analysis of techno-economic analysis studies. Renew. Sustain. Energy Rev. 2021, 145, 111098.
- Schuster, B.G.; Chinn, M.S. Consolidated bioprocessing of lignocellulosic feedstocks for ethanol fuel production. BioEnergy Res. 2013, 6, 416–435.
- Davison, S.A.; Keller, N.T.; van Zyl, W.H.; den Haan, R. Improved cellulase expression in diploid yeast strains enhanced consolidated bioprocessing of pretreated corn residues. Enzym. Microb. Technol. 2019, 131, 109382.
- Wilkinson, S.; Smart, K.A.; James, S.; Cook, D.J. Bioethanol production from brewers spent grains using a fungal consolidated bioprocessing (CBP) approach. Bioenergy Res. 2017, 10, 146–157.
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53.
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing theireffects. Bioresour. Technol. 2016, 199, 103–112.
- Šantek, M.I.; Grubišić, M.; Perečinec, M.G.; Beluhan, S.; Šantek, B. Lipid production by Mortierella isabellina from pretreated corn cobs and effect of lignocellulose derived inhibitors on growth and lipid synthesis. Process Biochem. 2021, 109, 46–58.
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16.
- Saini, R.; Kaur, A.; Saini, J.K.; Patel, A.K.; Varjani, S.; Chen, C.W.; Singhania, R.R.; Dong, C.D. Trends in Lignin Biotransformations for Bio-Based Products and Energy Applications. Bioenergy Res. 2022, 16, 88–104.
- Sukhang, S.; Choojit, S.; Reungpeerakul, T.; Sangwichien, C. Bioethanol production from oil palm empty fruit bunch with SSF and SHF processes using Kluyveromyces marxianus yeast. Cellulose 2020, 27, 301–314.
- Ballesteros, M.; Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I. Ethanol from lignocellulosic materials by a simulataneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem. 2004, 39, 1843–1848.
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Chaiyaso, T. Bioethanol production from cellulose-rich corncob residue by the thermotolerant Saccharomyces cerevisiae TC-5. J. Fungi 2021, 7, 547.
- Tomás-Pejó, E.; Oliva, J.M.; González, A.; Ballesteros, I.; Ballesteros, M. Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CECT 10875 in a simultaneous saccharification and fermentation fed-batch process. Fuel 2009, 88, 2142–2147.
- ASTM. Standard Specification for Denatured Fuel Ethanol for Blending with Gasolines for Use as Automotive Spark-Ignition Fuel. In Annual Book of ASTM Standards; ASTM Standard D4806-11; ASTM International: West Conshohocken, PA, USA, 2011.
- EN 15376; Automotive Fuels—Ethanol as a Blending Component for Petrol—Requirements and Test Methods. European Committee for Standarization: Brussels, Belgium, 2007.
- Zhao, X.Q.; Zi, L.H.; Bai, F.W.; Lin, H.L.; Hao, X.M.; Yue, G.J.; Ho, N.W.Y. Bioethanol from lignocellulosic biomass. Adv. Biochem. Eng. Biotechnol. 2012, 128, 25–51.
- Huang, H.J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21.
- Kunnakorn, D.; Rirksomboon, T.; Siemanond, K.; Aungkavattana, P.; Kuanchertchoo, N.; Chuntanalerg, P.; Wongkasemjit, S. Techno-economic comparison of energy usage between azeotropic distillation and hybrid system for water-ethanol separation. Renew. Energy 2013, 51, 310–316.
- Luyben, W.L. Distillation Economic Optimization. Distillation Design and Control Using Aspen TM Simulation; Wiley-Blackwell: Hoboken, NJ, USA, 2006; pp. 85–97.
- Luyben, W.L. Control of a multiunit heterogeneous azeotropic distillation process. AIChE J. 2006, 52, 623–637.
- Zhao, L.; Lyu, X.; Wang, W.; Shan, J.; Qiu, T. Comparison of heterogeneous azeotropic distillation and extractive distillation methods for ternary azeotrope ethanol/toluene/water separation. Comput. Chem. Eng. 2017, 100, 27–37.
- Popescu, A.E.P.; Pellin, J.L.; Bonet, J.; Llorens, J. Bioethanol dehydration and mixing by heterogeneous azeotropic distillation. J. Clean. Prod. 2021, 320, 128810.
- Gerbaud, V.; Rodriguez-Donis, I.; Hegely, L.; Lang, P.; Denes, F.; You, X. Review of extractive distillation. Process design, operation, optimization and control. Chem. Eng. Res. Des. 2019, 141, 229–271.
- Meirelles, A.; Weiss, S.; Herfurth, H. Ethanol dehydration by extractive distillation. J. Chem. Technol. Biotechnol. 1992, 53, 181–188.
- García-Herreros, P.; Gómez, J.M.; Gil, I.D.; Rodríguez, G. Optimization of the design and operation of an extractive distillation system for the production of fuel grade ethanol using glycerol as entrainer. Ind. Eng. Chem. Res. 2011, 50, 3977–3985.
- de Jesús Hernández-Hernández, E.; Cabrera-Ruiz, J.; Hernández-Escoto, H.; Gutiérrez-Antonio, C.; Hernández, S. Simulation study of the production of high purity ethanol using extractive distillation: Revisiting the use of inorganic salts. Chem. Eng. Process. Process Intensif. 2022, 170, 108670.
- Martínez-Galmiche, I.F.; Ramírez-Corona, N.; Conde-Mejía, C.; Sánchez-Sánchez, K.B.; Gani, R.; Jiménez-Gutiérrez, A. Design of energy-efficient ionic liquid-based extractive distillation systems for ethanol dehydration including alternatives for ionic liquid recovery. Chem. Eng. Res. Des. 2022, 188, 238–248.
- Fadia, G.; Hassiba, B.; Weifeng, S. Separation of ethanol–water mixture by extractive distillation using pyridinium-based ionic liquid 1-ethyl-3-methylpyridinium ethylsulfate. Chem. Eng. Process. Process Intensif. 2022, 173, 108815.
- Seiler, M.; Köhler, D.; Arlt, W. Hyperbranched polymers: New selective solvents for extractive distillation and solvent extraction. Sep. Purif. Technol. 2003, 30, 179–197.
- Li, G.; Liu, S.; Yu, G.; Dai, C.; Lei, Z. Extractive distillation using ionic liquids-based mixed solvents combined with dividing wall column. Sep. Purif. Technol. 2021, 269, 118713.
- Karimi, S.; Karri, R.R.; Tavakkoli Yaraki, M.; Koduru, J.R. Processes and separation technologies for the production of fuel-grade bioethanol: A review. Environ. Chem. Lett. 2021, 19, 2873–2890.
- Lei, Z.; Xi, X.; Dai, C.; Zhu, J.; Chen, B. Extractive distillation with the mixture of ionic liquid and solid inorganic salt as entrainers. AIChE J. 2014, 60, 2994–3004.
- Iqbal, A.; Ahmad, S.A. Pressure swing distillation of azeotropic mixture–A simulation study. Perspect. Sci. 2016, 8, 4–6.
- Sowerby, B.; Crittenden, B.D. An experimental comparison of type A molecular sieves for drying the ethanol-water azeotrope. Gas Sep. Purif. 1988, 2, 77–83.
- Wang, Y. Measurements and modeling of water adsorption isotherms of zeolite linde-type A crystals. Ind. Eng. Chem. Res. 2020, 59, 8304–8314.
- Adnađević, B.; Mojović, Z.; Abu Rabi, A. The kinetics of ethanol adsorption from the aqueous phase onto zeolite NaZSM-5. Adsorption 2008, 14, 123–131.
- Chaibi, A.; Boucheffa, Y.; Bendjaballah-Lalaoui, N. TGA investigation of water and ethanol adsorption over LTA zeolites. Microporous Mesoporous Mater. 2021, 324, 111285.
- Hajilari, M.; Shariati, A.; Khosravi-Nikou, M. Equilibrium adsorption of bioethanol from aqueous solution by synthesized silicalite adsorbents: Experimental and modeling. Adsorption 2019, 25, 13–31.
- Hajilari, M.; Shariati, A.; Khosravi-Nikou, M. Mass transfer determination of ethanol adsorption on activated carbon: Kinetic adsorption modeling. Heat Mass Transf. 2019, 55, 2165–2171.
- Laksmono, J.A.; Sudibandriyo, M.; Saputra, A.H.; Haryono, A. Structured polyvinyl alcohol/zeolite/carbon composites prepared using supercritical fluid extraction techniques as adsorbent for bioethanol dehydration. Int. J. Chem. Eng. 2019, 2019, 6036479.
- de Luna, M.D.G.; Divinagracia, M.F.; Choi, A.E.S.; Ong, D.C.; Chung, T.W. Applicability of composite silica–divinylbenzene in bioethanol dehydration: Equilibrium, kinetic, thermodynamic, and regeneration analysis. Energy Fuels 2019, 33, 7347–7356.
- Tang, Y.; Tanase, S. Water-alcohol adsorptive separations using metal-organic frameworks and their composites as adsorbents. Microporous Mesoporous Mater. 2020, 295, 109946.
- Claessens, B.; De Staercke, M.; Verstraete, E.; Baron, G.V.; Cousin-Saint-Remi, J.; Denayer, J.F. Identifying selective adsorbents for the recovery of renewable isobutanol. ACS Sustain. Chem. Eng. 2020, 8, 9115–9124.
- Ranjbar, Z.; Tajallipour, M.; Niu, C.H.; Dalai, A.K. Water removal from ethanol vapor by adsorption on canola meal after protein extraction. Ind. Eng. Chem. Res. 2013, 52, 14429–14440.
- Ghanbari, S.; Niu, C.H. Characterization of a high-performance biosorbent for natural gas dehydration. Energy Fuels 2018, 32, 11979–11990.
- Li, H.; Liu, Y.; Gao, X.; Li, X. Preparation and characterization of cassava starch-based adsorbents for separating of azeotropic ethanol-water in biofuels ethanol production. J. Chem. Technol. Biotechnol. 2016, 91, 977–984.
- Kularathne, I.W.; Gunathilake, C.; Yatipanthalawa, B.S.; Kalpage, C.S.; Rathneweera, A.C.; Rajapakse, S. Production of green energy–ethanol dehydration using rice straw, rice husk and castor oil. Biomass Convers. Biorefinery 2021, 11, 1597–1610.
- Einicke, W.D.; Gläser, B.; Schöoullner, R. In-situ recovery of ethanol from fermentation broth by hydrophobic adsorbents. Acta Biotechnol. 1991, 11, 353–358.
- Shirazi, M.M.A.; Kargari, A.; Tabatabaei, M. Sweeping gas membrane distillation (SGMD) as an alternative for integration of bioethanol processing: Study on a commercial membrane and operating parameters. Chem. Eng. Commun. 2015, 202, 457–466.
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for bioethanol production by pervaporation. Biotechnol. Biofuels 2021, 14, 10.
- Vane, L.M. Review: Membrane materials for the removal of water from industrial solvents by pervaporation and vapor permeation. J. Chem. Technol. Biotechnol. 2019, 94, 343–365.
- Gupta, O.; Roy, S.; Rao, L.; Mitra, S. Graphene Oxide-Carbon Nanotube (GO-CNT) Hy-brid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol. Membranes 2022, 12, 1227.
- Hietaharju, J.; Kangas, J.; Tanskanen, J. Analysis of the permeation behavior of ethanol/water mixtures through a polydimethylsiloxane (PDMS) membrane in pervaporation and vapor permeation conditions. Sep. Purif. Technol. 2019, 227, 115738.
- Saha, K.; Maharana, A.; Sikder, J.; Chakraborty, S.; Curcio, S.; Drioli, E. Continuous production of bioethanol from sugarcane bagasse and downstream purification using membrane integrated bioreactor. Catal. Today 2019, 331, 68–77.
- Chiao, Y.H.; Mai, Z.; Hung, W.S.; Matsuyama, H. Osmotically assisted solvent reverse osmosis membrane for dewatering of aqueous ethanol solution. J. Membr. Sci. 2023, 672, 121434.
- Zhang, X.; Ning, Z.; Wang, D.K.; Diniz da Costa, J.C. A novel ethanol dehydration process by forward osmosis. Chem. Eng. J. 2013, 232, 397–404.
- Wei, P.; Cheng, L.H.; Zhang, L.; Xu, X.H.; Chen, H.L.; Gao, C.J. A review of membrane technology for bioethanol production. Renew. Sustain. Energy Rev. 2014, 30, 388–400.
- Peng, P.; Shi, B.; Lan, Y. A review of membrane materials for ethanol recovery by pervaporation. Sep. Sci. Technol. 2010, 46, 234–246.
- Wee, S.L.; Tye, C.T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516.
- Lin, Y.S. Microporous and dense inorganic membranes: Current status and prospective. Sep. Purif. Technol. 2001, 25, 39–55.
- Claes, R.; Vandezande, P.; Mullens, S.; Sitter, K.D.; Peeters, R.; Van Bael, M.K. Preparation and benchmarking of thin film supported PTMSP-silica pervaporation membranes. J. Membr. Sci. 2012, 389, 265–271.
- Cheng, X.Q.; Konstas, K.; Doherty, C.M.; Wood, C.D.; Mulet, X.; Xie, Z.; Ng, D.; Hill, M.R.; Lau, C.H.; Shao, L. Organic microporous nanofllers with unique alcohol afnity for superior ethanol recovery toward sustainable biofuels. ChemSusChem 2017, 10, 1887–1891.
- Liu, Q.; Li, Y.; Li, Q.; Liu, G.; Liu, G.; Jin, W. Mixed-matrix hollow fiber composite membranes comprising of PEBA and MOF for pervaporation separation of ethanol/water mixtures. Sep. Purif. Technol. 2019, 214, 2–10.
- Liu, X.; Li, Y.; Zhu, G.; Ban, Y.; Xu, L.; Yang, W. An organophilic pervaporation membrane derived from metal–organic framework nanoparticles for efficient recovery of bio-alcohols. Angew. Chem. Int. Ed. 2011, 50, 10636–10639.
- Liu, X.; Hu, D.; Li, M.; Zhang, J.; Zhu, Z.; Zeng, G.; Zhang, Y.; Sun, Y. Preparation and characterization of Silicalite-1/PDMS surface sieving pervaporation membrane for separation of ethanol/water mixture. J. Appl. Polym. Sci. 2015, 132, 42460.
- Tang, X.; Wang, R.; Xiao, Z.; Shi, E.; Yang, J. Preparation and pervaporation performances of fumed-silica-filled polydimethylsiloxane–polyamide (PA) composite membranes. J. Appl. Polym. Sci. 2007, 105, 3132–3137.
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; van der Bruggen, B. Metal–organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144.
- Pan, Y.; Yu, X. Preparation of Zeolitic Imidazolate Framework-91 and its modeling for pervaporation separation of water/ethanol mixtures. Sep. Purif. Technol. 2020, 237, 116330.
- Shafiei Amrei, S.; Asghari, M.; Esfahanian, M.; Zahraei, Z. Highly selective carbon nanotube-coupled graphene oxide-incorporated polydimethylsiloxane membrane for pervaporative membrane bioreactor ethanol production. J. Chem. Technol. Biotechnol. 2020, 95, 1604–1613.
- Li, C.; Li, J.; Cai, P.; Cao, T.; Zhang, N.; Wang, N.; An, Q.F. Liquid–liquid interface induced PDMS-PTFE composite membrane for ethanol perm-selective pervaporation. AIChE J. 2020, 68, e17694.
- Kim, H.J.; Kim, S.J.; Lee, K.; Foster, R.I. A short review on hydrophobic pervaporative inorganic membranes for ethanol/water separation applications. Korean J. Chem. Eng. 2022, 39, 2263–2274.
- Huang, H.; Qureshi, N.; Chen, M.H.; Liu, W.; Singh, V. Ethanol production from food waste at high solids content with vacuum recovery technology. J. Agric. Food Chem. 2015, 63, 2760–2766.
- Roffler, S.R.; Blanch, H.W.; Wilke, C.R. In situ recovery of fermentation products. Trends Biotechnol. 1984, 2, 129–136.
- Rivera, E.C.; Atala, D.I.P.; Filho, F.M.; Carvalho da Costa, A.; Filho, R.M. Development of real-time state estimators for reaction–separation processes: A continuous flash fermentation as a study case. Chem. Eng. Process. Process Intensif. 2010, 49, 402–409.
- Wang, Y.Z.; Liao, Q.; Lv, F.L.; Zhu, X.; Ran, Y.; Hou, C.J. Solid simultaneous saccharification and fermentation of rice straw for bioethanol production using nitrogen gas stripping. RSC Adv. 2015, 5, 55328–55335.
- Jin, M.; Sarks, C.; Bals, B.D.; Posawatz, N.; Gunawan, C.; Dale, B.E.; Balan, V. Toward high solids loading process for lignocellulosic biofuel production at a low cost. Biotechnol. Bioeng. 2017, 114, 980–989.
- Jones, R.A.; Gandier, J.A.; Thibault, J.; Tezel, F.H. Enhanced ethanol production through selective adsorption in bacterial fermentation. Biotechnol. Bioprocess Eng. 2011, 16, 531–541.
- Hashi, M.; Thibault, J.; Tezel, F.H. Recovery of Ethanol from Carbon Dioxide Stripped Vapor Mixture: Adsorption Prediction and Modeling. Ind. Eng. Chem. Res. 2010, 49, 8733–8740.
- Seo, D.J.; Takenaka, A.; Fujita, H.; Mochidzuki, K.; Sakoda, A. Practical considerations for a simple ethanol concentration from a fermentation broth via a single adsorptive process using molecular-sieving carbon. Renew. Energy 2018, 118, 257–264.
- Matsumura, M.; Märkl, H. Application of solvent extraction to ethanol fermentation. Appl. Microbiol. Biotechnol. 1984, 20, 371–377.
- Job, C.; Schertler, C.; Staudenbauer, W.L.; Blass, E. Selection of organic solvents for in situ extraction of fermentation products from Clostridium thermohydrosulfuricum cultures. Biotechnol. Tech. 1989, 3, 315–320.
- Munson, C.L.; King, C.J. Factors influencing solvent selection for extraction of ethanol from aqueous solutions. Ind. Eng. Chem. Process Des. Dev. 1984, 23, 109–115.
- Kollerup, F.; Daugulis, A.J. Ethanol production by extractive fermentation-solvent identification and prototype development. Can. J. Chem. Eng. 1986, 64, 598–606.
- Widjaja, T.; Altway, A.; Permanasari, A.R.; Gunawan, S. Production of Ethanol as A Renewable Energy by Extractive Fermentation. Appl. Mech. Mater. 2014, 493, 300–305.
- Fan, S.; Liu, J.; Tang, X.; Wang, W.; Xiao, Z.; Qiu, B.; Wang, Y.; Jian, S.; Qin, Y.; Wang, Y. Process operation performance of PDMS membrane pervaporation coupled with fermentation for efficient bioethanol production. Chin. J. Chem. Eng. 2019, 27, 1339–1347.
- Serna-Vázquez, J.; Zamidi Ahmad, M.; Castro-Muñoz, R. Simultaneous production and extraction of bio-chemicals produced from fermentations via pervaporation. Sep. Purif. Technol. 2021, 279, 119653.
- Maiorella, B.; Blanch, H.W.; Wilke, C.R. Rapid Ethanol Production. In Proceedings of the AIChE 72nd National Meeting, San Francisco, CA, USA, 25 November 1979; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1979.
- Zentou, H.; Abidin, Z.Z.; Yunus, R.; Biak, D.R.A.; Korelskiy, D. Overview of Alternative Ethanol Removal Techniques for Enhancing Bioethanol Recovery from Fermentation Broth. Processes 2019, 7, 458.
- Olukman, M.; Şanlı, O.; Solak, E.K. Synthesis of magnetite in poly(vinyl alcohol) matrix and its use in separation of acetone/water mixtures via pervaporation, vapor permeation with and without temperature difference methods. Vacuum 2015, 120, 107–115.
- Vane, L.M.; Alvarez, F.R. Membrane-assisted vapor stripping: Energy efficient hybrid distillation-vapor permeation process for alcohol-water separation. J. Chem. Technol. Biotechnol. 2008, 83, 1275–1287.
More
