1. Introduction
Every year, contaminated food is responsible for 420,000 deaths and 600 million cases of foodborne illnesses caused by spoiled food
[1]. This is not just a problem in low–middle-income countries, high-income countries also have several troubles related to foodborne pathogens. In the U.S. alone, there are more than 9.4 million deaths per year due to the ingestion of pathogenic bacteria in food
[2]. During 2010, 420,000 people (one-third of them being children under the age of five) died from illnesses related to salmonellosis and
Escherichia coli infections
[3]. Foodborne illnesses arise from the presence of pathogens, toxins, or contaminants in food products, and are typically associated with gastrointestinal symptoms (diarrhea, vomiting, abdominal pain, and fever), and other adverse effects on human health such as neurological, hepatic, and renal complications, even becoming a life-threatening issue if not appropriately addressed
[4][5][4,5]. In recent years, the majority of reported foodborne illness outbreaks were caused by pathogens such as Norovirus
[5],
Campylobacter [6],
Salmonella [5][6][5,6],
Listeria monocytogenes [7], and Shiga toxin-producing
E. coli [8]. Less frequently reported but still of concern are the pathogens
Staphylococcus aureus [9],
Clostridium species
[10],
Bacillus cereus [11], and
Yersinia enterocolitica [12].
Similarly to food safety, the presence of pathogens in water is a major issue for public health
[13]. It is estimated that 663 million people consume unsafe water from surface or groundwater sources
[14]. More than 2.2 million deaths per year and more cases of illness (diarrhea, gastrointestinal, and systematic diseases) are linked to contaminated water ingestion
[15]; the pathogens of greatest concern are
Salmonella,
Shigella,
Campylobacter,
S. aureus, and
E. coli [16][17][16,17]. However, viruses and parasites are becoming a problem for water security
[18]. Parasites and viruses linked to waterborne outbreaks include
Vibrio cholerae,
Leptospira,
Schistosoma mansoni, and
Schistosoma japonicum [16][19][20][16,19,20].
Monitoring the presence of pathogens in water is particularly important as a disease-preventive measure from waterborne illnesses and to monitor water quality. This can be achieved through applying wastewater-based surveillance protocols, which allow the detection of pathogens using molecular biology tools
[21][22][21,22], which can be applied to verify the discharged water quality and indicate the treatment required to prevent adverse effects on the environment; ensuring water sustainability for future generations.
Pathogen-detection methods play a crucial role in ensuring food and water safety; however, actual monitoring methods are time-consuming processes that usually take days to obtain a precise result
[23], making them ineffective for real-time monitoring
[24]. In fact, the identification of pathogens such as bacteria and viruses is carried out by gold-standard methodologies, which are traditional techniques such as viable plate counts, flow cytometry, and staining methods, among others
[25][26][27][25,26,27]. Nevertheless, the detection time is one of the major limitations of this technique because these techniques require the growth of the microorganism in laboratory conditions (this has not been a limitation per se), which can take several days to produce a result, hindering the response time for the control of pathogens
[26]. Techniques based on molecular biology that are used for pathogen detection involve
[28] polymerase chain reaction techniques (PCR)
[21][29][30][31][21,29,30,31], multiplex polymerase chain reaction (mPCR)
[32], quantitative polymerase chain reaction (qPCR)
[33], digital droplet PCR (ddPCR)
[34], fluorescence in situ hybridization (FISH)
[31], enzyme-linked immunosorbent assay (ELISA)
[35], surface-enhanced Raman spectroscopy (SERS)
[36], immunological methods
[37], next-generation sequencing
[38], whole-genome sequencing
[39], flow cytometry
[40], and surface plasmon resonance imaging (SPR)
[41]; these techniques have already been applied as detection methodologies of pathogens in food and water matrices
[5][26][5,26].
Despite the application of molecular-biology techniques in food and water security, if
rwe
searchers consider the technological development of the health sector related to pathogen detection, this sector has already developed advanced technologies such as biosensors with nanomaterials and the incorporation of informatic technologies
[42]. Efforts are being conducted in the hope of bringing about more specific and faster methodologies to produce a rapid-response diagnosis and prevent outbreaks, focusing on nanomaterials such as glyconanomaterials
[43], nanoparticles
[44], ZnO nanorods, nanoconjugate (Au–Fe
3O
4), silicon nanonet FET, nanosphere (RNs@Au) in a biosensor device, combined with molecular detection methods (ELISA, qPCR) and also incorporated with informatic technologies, which are used to create more-sensible and appropriate in situ detection systems for pathogens of major concern. This technology has been applied in order to improve the health care system’s response to pathogen-presence emergencies, (as reviewed by Jian et al., 2021)
[45] for HIV and Influenza A virus. These technologies have also been applied to Ebola
[46], Malaria
[47], Dengue virus
[43], and in recent years in SARS-CoV-2 monitoring protocols
[42]. Considering the advances made in health security and the demands for improved food and water safety, these existing technologies in the health sector should be transferred to other sectors such as food and water security.
For the above mentioned, and the increase in pathogens related to food and water-borne illnesses, the development of pathogen-detection methods is becoming an urgent step to ensuring health and safety
[48]. Unfortunately, and despite recent advances in new pathogen-detection approaches, the application of nanomaterials and biosensors is still limited, this is why technologies capable of obtaining better results, in a fast and affordable way, have been studied, resulting in novel technologies, such as biosensor devices, with “rapid, sensitive and specific” protocol for pathogen detection, resolving the priority assignment of ensuring health security, preventing food- and water-ingestion-related outbreaks
[49], with even more affordable technology with the inclusion of the use of biosensors and NPs in recent years
[44][50][51][44,50,51].
The previously mentioned methods help to perform faster monitoring (real-time surveillance systems)
[52], reducing response times of pathogen detection in water
[53]. Additionally, the use of biosensors improved with NPs enhanced the detection performance of the device making it a faster, more specific, and portable device
[54]. In fact, due to the diversity of the detection capabilities of nanoparticles, they are the subject of many studies that attempt to understand their role when incorporated into pathogen-detection systems
[55].
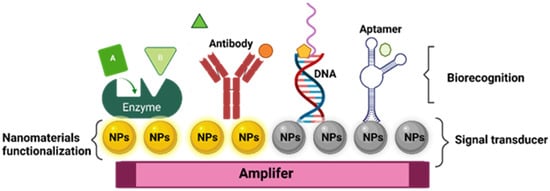
The basic components of a biosensor device are a biorecognition element, a transducer, an amplifier, and a processor component. The biorecognition element recognizes the analyte of interest, the transducer generates a signal from the recognition of the biomarker into a measurable signal, then the signal is processed using the processor and amplifier component, to obtain a signal output
[56][57][56,57]. In summary, it is a bioanalytical device that detects specific biomarkers using biochemical reactions
[58], mediated by isolated enzymes, antibodies, tissues, organelles, or whole cells for pathogen detection, using electrical, thermal, or optical signals
[59], which are able to correlate the presence of specific pathogen and signal emission measures
[50].
As is already mentioned, the biosensor application has garnered attention in the field of pathogen detection due to their attractive characteristics, such as precision, selectivity and fast analysis
[27]. Nevertheless, it is necessary to mention that these methodologies have certain disadvantages, such as the use of expensive enzymes and equipment, including the extensive workflow required for the device’s development. However, these technologies have a promising future due to their potential application in pathogen-rapid-detection methods
[53]. Currently, biosensor-based technology has proved its worth due to its unique sensitivity, low detection limit, and simple operation
[60].
In the last decade, the biosensors’ structure has been focused on the miniaturization of the devices without affecting the detection efficacy. To achieve this, NPs have been included in the biosensor architecture, resulting in the development of a nanoscale platform. Indeed, in the different sections of the biosensor, NPs are used as signal transducers to convert a biomolecular interaction into an electrical, optical, or magnetic signal
[61]. This functionality inside the biosensor is because of unique properties at the nanometric scales (surface area, small size, affinity for some biomolecules, catalytic activity, and autofluorescence)
[62][63][62,63].
Like traditional biosensor devices, the nanobiosensors are composed of three main sections: a biorecognition probe, transducer, and amplifier
[64] (
Figure 1). The NPs are often in the transducer’s component, helping to enhance the biochemical, electrical, magnetic, or optical signal transduction
[61]. Also, these signals can be read simply and effectively as a result of the incorporation of functionalized NPs into the biorecognition component
[65].
Figure 1.
General structure of nanobiosensor with different agents of biorecognition.
In fact, nanomaterials have been identified as candidates to enhance biosensors’ sensitivity, improving the detection limits and increasing detection specificity
[54][55][54,55]. The foregoing is based on the fact that the specificity of signal recognition results in the adequate selection of functionalized ligands with NPs, improving the biomarker attraction; also, NPs convert signals from one form to another or act as detectors of the generated signals
[66][67][66,67]. Biosensors have several methodologies to acquire relevant signals; for example, the electrochemical biosensors work under the method of capitalizing on reactions between immobilized biomolecules and the biomarker, resulting in electron/ion generation/consumption, modifying the electrical properties of the solution, and resulting in a measurable electrical current
[68]. On the other hand, optical biosensors work under the method of discerning variations in light properties (absorption, transmission, and reflection), triggered by physical or chemical interactions with biorecognition elements. These biosensors are categorized into two major groups: label-free, where signals arise directly from analyte interactions, and label-based, employing techniques such as calorimetry, fluorescence, or luminescence to produce detectable optical signals. Both methodologies are available to be applied in diverse areas for pathogen detection
[69][70][71][69,70,71].
Other possible classifications of biosensors are based on the type of biorecognition immobilized on the nanomaterial
[72], which is divided into the following: enzymes
[73], antibodies
[74], antigens
[75], DNA-RNA
[76], organelle
[77], cell membrane
[78], and phage particle
[79]. The conversion of this signal can be achieved using different methods, and this can be classified according to the type of conversion used
[80]. Finally, the signal conversion section can include the following optical systems:
[69] electrochemical nanobiosensors
[81], thermoelectric
[82], and piezoelectric
[83].
2. Nanomaterials for the Detection of Pathogens in Water and Food
As is mentioned above, one of the major concerns in food and water safety is the precise detection of pathogens, this has led, in combination with novel sensor technologies, to an increasing exploration of nanomaterials in combination with highly efficient aptamers to revolutionize the pathogen detection in water and food. This fusion of nanotechnology and aptamers opens new possibilities for more effective control and quicker responses to potential public health risks. The following Table 1 summarizes the last five years of nanobiosensor production for the detection of viruses, bacteria, and parasites using aptamers in complex matrices.
Table 1. NPs application for detection of pathogenic bacteria in food and water matrices.
| Nanomaterial |
Pathogen |
Matrix |
LOD |
Signal |
Bioconjugate Material |
Reference |
| Iron core gold NPs |
S. enteritidis |
Beverage samples |
32 Salmonella mL−1 |
Fluorescence |
Antibody |
[63] |
| FeO-NPS and Quantum dots |
E. coli |
Water |
1 × 102 CFU |
Fluorescence |
Aptamer |
[84][122] |
| NAC (N-acetylcysteine) monomer |
L. monocytogenes |
Milk and pork meat |
1 × 103 CFU mL−1 |
Fluorescence |
MPIs |
[85][131] |
| Au-N triangles |
P. aeruginosa |
Water |
1 cell |
LSPR |
Aptamer |
[86][134] |
| Ag-NPs |
E. coli |
Water |
150 CFU mL−1 |
Electrochemical |
Aptamer |
[87][135] |
| AgNPs |
S. aureus |
Bacterial suspension and human serum |
1.0 CFU mL−1 |
Electrochemical |
Aptamer |
[88][136] |
| Au-NPs |
S. aureus |
Tap water |
101 to 104 CFU mL−1 |
Fluorescence |
Aptamer |
[89][141] |
| AuNPs |
S. aureus |
Luria-Bertani media |
1.5 × 107 cells mL−1 |
Colorimetric |
Aptamer |
[90][142] |
| AuNPs |
Ochratoxin A |
Peanut, soybean, and corn |
28.18 pg/mL |
Colorimetric |
Aptamer |
[91][143] |
| AuNPs |
E. coli |
Flour |
2.5 ng µL−1 |
Colorimetric |
Probe |
[92][144] |
Graphene
oxide coated AuNPs |
E. coli
S. Typhimurium |
Bacterial suspension |
1 × 103 CFU |
Colorimetric |
Antibody |
[93][145] |
| Ag-NPs |
S. aureus |
Water |
1.0 CFU mL−1 |
Electrochemical |
Aptamer |
[94][146] |
| Chitosan-AgNPs |
Glipopolysaccharide |
Bacterial suspension |
248 CFU mL−1 |
Electrochemical |
- |
[95][147] |
| AgNPs |
E. coli |
Pork, cabbage and milk |
2.0 CFU mL−1 |
Photoelectrochemical |
Peptide Magainin |
[96][148] |
| Au-NPs and oxide of graphene NPs |
E. coli |
Water |
9.34 CFU mL−1 |
Electrochemical |
Aptamer |
[97][149] |
| Multiwalled carbon nanotubes |
E. coli |
Water |
0.8 CFU mL−1 |
Electrochemical |
Antibody |
[98][150] |
| Graphene and carbon nanotubes |
Salmonella enteritidis |
Water |
102–108 CFU mL−1 |
Colorimetric |
Antibody |
[99][151] |
| Quantum dots |
S. aureus, S. Typhimurium |
Water |
16–28 CFU mL−1 |
Colorimetric |
Aptamers |
[100][152] |
| SiNPs |
E. coli |
Bacterial suspension |
103 CFU mL−1 |
Electrochemical |
Polyclonalantibodies |
[101][153] |
| SiNPs |
E. coli |
Bacterial suspension |
8 CFU mL−1 |
Fluorescence |
Rhodamine B |
[102][154] |
| SiNPs |
AFB1 from filamentous fungi |
Peanut, maize, and badam |
0.214 pg mL−1 |
Fluorescence |
Aptamer |
[103][155] |
| MNPs |
S. aureus |
Milk, Romaine lettuce, ham, and sausage |
2.5 ng
µL−1 |
Colorimetric |
Probes |
[104][156] |
| Iron oxide MNPs assisted AuNPs |
B. cereus and Shigella flexneri |
Inoculated media |
12 cells mL−1 and
3 cells mL−1 |
Electrochemical |
Vancomycin |
[105][157] |
| Magnetic NPs |
S. Typhimurium |
Food |
53 UFC/mL |
Fluorescence |
Oligonucleotides |
[106][158] |
| Iron oxide encapsulated quantum dots |
Hepatitis E virus
Norovirus |
Clinical samples |
56 RNA copies mL−1
69 RNA copies mL−1 |
Fluorescence
Electrochemical |
Antibody |
[107][159] |
| QDs |
S. Typhimurium |
Chicken meats |
43 CFU mL−1 |
Fluorescence |
Antibody |
[108][160] |
| QDs |
S. Typhimurium and V. parahaemolyticus |
Aquatic samples |
10 CFU mL−1
102 CFU mL−1 |
Fluorescence |
Aptamer |
[109][161] |
| QDs nanobeads |
S. Typhimurium |
Potable water, orange juice, lettuce, and chicken |
10−1 CFU mL−1 |
Fluorescence |
Antibody |
[110][162] |
| TAA *, TBA **, TMA *** and TE **** |
S. aureus |
Lettuce/Shrimp |
4 CFU mL−1 |
Electrochemical/Fluorescence |
MPIs |
[111][163] |
Nanobiosensors, due to their small size and high sensitivity, enable the real-time detection of low concentrations of biomarkers, a crucial characteristic in applications of food and water monitoring. This versatility allows them to adapt to various molecules and technologies, such as artificial intelligence incorporation. Moreover, they are more cost-effective and environmentally friendly than conventional techniques. Their miniaturization capability makes them ideal for portable devices and on-site diagnostic systems, providing quick and efficient access to quality testing and analysis in food and water. This makes them promising tools in various scientific and technological applications (Figure 2).
Figure 2. Strengths of nanobiosensors.
4.1. Gold Nanopartícles (Au-NPs)
Among the different types of NPs, metallic nanoparticles (MNPs) exhibit many useful characteristics such as high surface-to-volume ratio, conductivity, selectivity, and excellent optical and chemical properties, for their application in the biotechnology field
[112][113][164,165]. The application can vary depending on the metal used, size, shape, surface properties, and functionalization of the MNPs
[114][166]. On one hand, Au-NPs have been successfully used in pathogen detection because they can easily be conjugated with recognition and biorecognition elements such as aptamers, DNA, antibodies, carbohydrates, and proteins, which can enhance the reactivity and selectivity of the NPs towards specific pathogens
[51][115][51,167].
In fact, Au-NPs are one of the most stable MNPs, not to mention their unique characteristics such as good chemical reactivity, conductivity, and high resistance, which have attracted attention for their use in biosensor development
[116][168]. The surface of Au-NPs has been functionalized with various biocomponents
[117][169]. These nanobiosensors have a very low LOD for different chemical and biological analytes, not to mention their high stability against oxidation
[116][168]. Also, their characteristics, such as stability, conjugation, amplification properties, and their ability to serve as colorimetric biosensors
[118][119][120][170,171,172] are especially relevant in the case of Au-NPs due to their localized surface plasmon resonance, which is a phenomenon that gives unique optical properties to MNPs, particularly Au-NPs. This is due to the interaction of electromagnetic waves with NPs of specific sizes and shapes, resulting in differential absorption of the light spectrum and different colors exhibited by the NPs
[50][51][50,51]. These properties can be altered in the presence of different analytes, making Au-NPs highly suitable for biosensor development.
4.2. Silver Nanoparticles (Ag-NPs)
Ag-NPs stand out for their wide range of applications. These nanomaterials have been incorporated into textiles, healthcare products, consumer goods, medical devices, and biodetection applications, among others
[121][173]. These materials are highly attractive in diagnostics field due to high conductivity, catalytic activity, and plasmonic properties presented, which may be leveraged to enhance the biosensor’s performance
[122][174]. Sensitivity is a crucial factor for biosensors to detect low concentrations of biomarkers. Ag-NPs have been used to increase the electroactive surface area of electrodes, enhancing the electron-transfer rate and improving biosensor sensitivity
[121][122][173,174]. In the incorporation of Ag-NPs in biosensor structures, Ag-NPs can amplify signals or improve the detection of nucleic acids. Their plasmonic resonance absorption band, below 500 nm, confers selective absorption in the visible and near-infrared spectrum
[116][168]. In connection with pathogen detection, the phenomenon of surface plasmon resonance (SPR) works using the electrons on the surface of a metal, which are excited by photons of specific wavelengths and incidence angles
[123][124][175,176] and applied to target detection based on the refractive index
[123][175]. This is achieved when the biomarker is bound to a biorecognition element of the biosensor, the recognition event between the biomarker and the biorecognition element results in a change in the SPR resonance angle
[31]. Conjugated polymers, such as those that include silver nanoparticles are promising materials for addressing the current and emerging issues such as pandemic monitoring
[125][177], and pathogen detection both in food
[96][148] and water
[94][146].
4.3. Carbon-Based Nanoparticles
Similar to Au-NPs, carbon-based NPs are useful for the implementation of detection techniques for pathogen monitoring in water
[126][127][119,178]. Carbon-based NPs such as carbon nanotubes, graphene, and carbon nanodots have great potential in the biosensing of pathogens because of their ability to be coated with different biomolecules for the association of molecular patterns from pathogens and to generate a signal for specific pathogens as functionalized NPs can mimic the specific surface structure of pathogens
[128][179]. Carbon NPs have been used in the fluorescence resonance energy transfer (FRET) technique with quantum dots as donors modified with aptamers for the detection of
Vibrio parahaemolyticus and
S. Typhimurium in the range of 25 to 35 CFU mL
−1 and up to between 50 and 10
6 CFU mL
−1, respectively
[129][180]. Also, these NPs can be used in combination with aptamers to amplify the sensitivity and specificity of the device.
4.4. Magnetic Nanomaterials (MNPs)
Magnetic NPs possess their own versatility when used for biosensing pathogens, because of their specific attributes, particularly fast separation and concentration, that makes them easy tools for pathogen detection
[130][181]. MNPs have been used for detecting pathogens using nucleic acid detection and quantification in devices for point-of-care testing in the detection of the Hepatitis B virus (LOD of 50 IU mL
−1) and SARS-CoV-2 (500 copies mL
−1)
[131][182]. Magnetic NPs (MNPs) can conform to a section of the transducer part of the biosensor, or be suspended in solution in direct contact with the analyte of interest
[132][183]. When the MNPs are in contact with the sample, they bind to the target molecule through the interaction of the label in the NPs (a functional group) and a protein; once the complex of MNPs and target is formed, an external magnetic field attracts it to the active-detection surface, and after a wash of the unbinding molecules, targets are detected
[133][184].
When talking about magnetic NPs in biosensing, it is important to mention the magnetic relaxation switching mechanism (MRS). This phenomenon describes the incidence when cross-linking occurs between the MNPs in the binding and recognition of targets. When these MNPs clusters are formed, a change in the transverse relaxation of the sample is reflected as motional averaging or static dephasing according to the MNPs cluster size and this change can be monitored using nuclear magnetic resonance
[134][185].
4.5. Silica Nanoparticles (Si-NPs)
Si-NPs have applications in the biomedical field
[135][186], and they present good optical properties and good biocompatibility
[136][187]. NPs are mesoporous, so in combination with other metals, have attractive and profitable characteristics for biosensing purposes
[137][188]. Their uniformity and easily changed pore size among the gating mechanism makes it very useful in biosensing for drug delivery, for example
[138][189]. Another important characteristic of Si-NPs is that they are considered as a GRAS (generally recognized as safe) material by the FDA
[139][140][190,191]. The mesoporous nature of the Si-NPs is characteristic of a large interest, this feature can be employed to separate bacteria from complex samples even preserving its viability, and colloidal stabilization of magnetic NPs for the same purpose. Also, the silanol functional groups from SiNPs make possible the use and design of various bio-recognition systems that help to increase their sensibility and selectivity while reducing the detection time of different pathogens
[139][190].
4.6. Quantum Dots (QD)
Quantum dots (QD) are colloidal nanocrystalline semiconductors that possess properties such as a quantum confinement effect, allowing them to emit and absorb light at specific wavelengths
[140][191]. Because of this, QDs exhibit excellent optical properties, including a broad absorption spectrum, a narrow emission spectrum, and tunable luminescence, which show great prospects in biodetection
[141][192]. QD-based biosensors include but may not be limited to fluorescence, bioluminescent, chemiluminescent, and photoelectrochemical approaches
[142][193]. Some of the characteristics that make the use of quantum dots attractive for biosensing applications are that they possess high-quantum yield, better photobleaching resistance, wide absorption spectra, a narrow emission spectrum and their specificity with biologic targets in comparison with common fluorophores and dyes
[143][194]. Also, it is very remarkable that its surface is easily functionalized with biologic components in order to integrate QD probes
[142][193]. In the field of nanomaterials, the use of combinations of magnetic compounds displays attractive characteristics for current applications; these nanocomposites, besides maintaining complementary magnetic behavior, add functional proprieties to the final product
[107][159].
As presented above, numerous studies focus their determinations on
S. Typhimurium mainly because it is the most common pathogen related to food poisoning in Western countries causing gastroenteritis
[144][195]. If well-used as the model or the target of the experimentations, the modifications in for example primers’ design or binding proteins may allow the replication of studies carried with this strain to any other food pathogens
[109][110][145][161,162,196].