DEAD-box decapping enzyme 开螟酶20 (DDX20) is a putative RNA-decapping enzyme that can be identified by the conserved motif Asp–Glu–Ala–Asp (DEAD). Cellular processes involve numerous RNA secondary structure alterations, including translation initiation, nuclear and mitochondrial splicing, and assembly of ribosomes and spliceosomes. DDX20 reportedly plays an important role in cellular transcription and post-transcriptional modifications. On the one hand, DDX20 can interact with various transcription factors and repress the transcriptional process. On the other hand, DDX20 forms the survival motor neuron complex and participates in the assembly of snRNP, ultimately affecting the RNA splicing process. Finally, DDX20 can potentially rely on its RNA-unwinding enzyme function to participate in (DDX20)是一种假定的RNA开荬酶,可以通过保守基序Asp-Glu-Ala-Asp(DEAD)进行鉴定。细胞过程涉及许多RNA二级结构改变,包括翻译起始,核和线粒体剪接以及核糖体和剪接体的组装。据报道,DDX20在细胞转录和转录后修饰中起重要作用。一方面,DDX20可以与各种转录因子相互作用并抑制转录过程。另一方面,DDX20形成存活运动神经元复合体并参与snRNP的组装,最终影响RNA剪接过程。最后,DDX20可以潜在地依靠其RNA解开酶功能参与microRNA (miRNA) maturation and act as a component of the RNA-induced silencing complex. (miRNA)成熟,并作为RNA诱导的沉默复合物的组分。
- DDX20
- NF-κB
- transcription
- SMN complexe
- cancer
- miRNA
1. Introduction简介
2. Distribution, Structure, and Subcellular Localization of DDX20的分布、结构和亚细胞定位
作为具有As a novel human member of the TP依赖性RNA解绕酶的DEAD-box family with ATP-dependent RNA-unwinding enzymes, DDX20 was detectable at the molecular level in mammalian cells with monoclonal antibodies against DDX20 elevated to 家族的新型人类成员,DDX20在哺乳动物细胞的分子水平上可检测到,针对DDX20的单克隆抗体已升高至103 kDa in size [6]. Subsequent studies revealed that the Cryptobacterium hidradenum gene [6]。随后的研究表明,编码DDX46蛋白的隐杆菌基因Mel-46 encoding the DDX20 protein is expressed throughout its development and plays a facilitating role during development [7]. The monitoring results at the cellular level revealed that decreased 在其整个发育过程中表达,并在发育过程中发挥促进作用[7]。细胞水平的监测结果显示,在肝细胞癌组织中可检测到DDX20 expression could be detected in cancerous tissues of hepatocytes, thus affecting the disease process [8][9]. In addition, at the tissue and organ level, 表达降低,从而影响疾病进程[8,9]。此外,在组织和器官水平上,DDX20 was expressed in the testis and even in steroid- and nonsteroid-producing tissues [10]. Furthermore, 在睾丸中表达,甚至在产生类固醇和非类固醇的组织中表达[10]。此外,DDX20 is significantly overexpressed in certain cancerous tissues, such as hepatocellular carcinoma and colorectal, prostate, and gastric cancers, and usually indicates a good prognosis [11][12][13][14]. 在某些癌组织中显著过表达,例如肝细胞癌和结直肠癌、前列腺癌和胃癌,通常表明预后良好[11,12,13,14]。DDX20 was first encoded by the genome of the parthenogenetic multicellular organism 首先由单性生殖多细胞生物Dictyostelium discoideum and is retained in postnatal animals [15]. Some drugs, suc的基因组编码,并保留在出生后动物中[15]。一些药物,如他汀类药物,也可以通过甲羟戊酸途径和Rh as statins, can also inhibit DDX20 expression through the mevalonate pathway and downstream of RhoA [16].A下游抑制DDX20的表达[16]。 The DEAD-box RNA-decapping enzyme family features a unique Asp–Glu–Ala–Asp (消解酶家族具有独特的Asp-Glu-Ala-Asp(D-E-A-D) sequence motif [17]. All )序列基序[17]。所有DEAD-box decapping enzymes, including 开盖酶(包括DDX20, possess a conserved core structural domain at the N-terminal end, primarily comprising two recombinase A (RecA)-like structural domains [18]. As shown in Figure )在N端都有一个保守的核心结构域,主要包括两个重组酶A(RecA)样结构域[18]。如图1, this core structural domain includes nine conserved motifs, namely 所示,该核心结构域包括1个保守基序,即Q, I, Ia, Ib, II, III, IV, V, and VI, which are involved in ATP binding and hydrolysis, RNA substrate binding, regulating decapping enzyme activity via ATP-binding hydrolysis, and regulating ATPase activity [1]. The 、I、Ia、Ib、II、III、IV、V和VI,它们参与ATP结合和水解、RNA底物结合、通过ATP结合水解调节开盖酶活性以及调节ATP酶活性[19]。D-E-A-D sequence序列基序主要存在于高度保守的基序 motif is primarily found in the highly conserved motif II, which is also the source of the II 中,这也是“DEAD-box” name. Nonconserved N- and C-terminal auxiliary domains flank the core domain, ranging in size from a few to several hundred amino acids. These are mainly associated with specific functions of the decapping enzyme [19]. For instance, the C-terminal region of 名称的来源。非保守的N端和C端辅助结构域位于核心结构域的两侧,大小从几个到几百个氨基酸不等。这些主要与开胃酶的特定功能有关[20]。例如,DDX20 is necessary for it to maintain its uncoupling helicase activity [20].的C端区域是维持其解偶联解旋酶活性所必需的[<>]。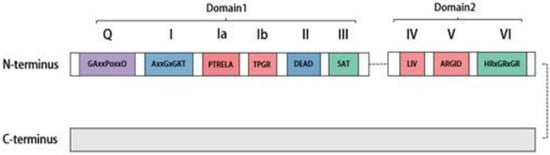
3. Splicing Features of DDX20的拼接特点
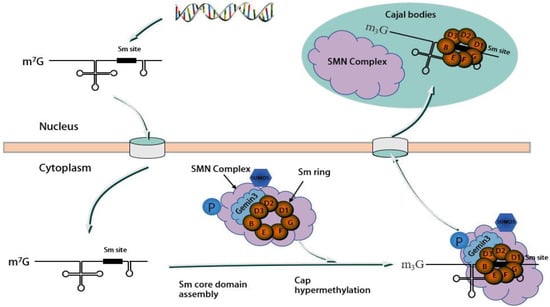
Existing research indicates that the motor neuron protein 现有研究表明,运动神经元蛋白SMN, along with 与Gemin2–8 proteins, including -8蛋白(包括DDX20/Gemin3 and Unrip, form a stable SMN complex, with 和Unrip)一起形成稳定的SMN复合物,其中DDX20/Gemin3 playing a vital role in the assembly of this complex [32][33][34]. The assembly process of the 在该复合物的组装中起着至关重要的作用[32,33,34]。SMN complex is modular, involving the physical association of several 复合物的组装过程是模块化的,涉及几种SMN complex proteins. 复合物蛋白质的物理结合。SMN/Gemin8/Gemin7, situated at the core of the complex, recruit other proteins to form the complete assembly [34].位于复合物的核心,募集其他蛋白质形成完整的组装体[34]。 As a molecular companion, the 作为分子伴侣,SMN complex facilitates the assembly and function of spliceosomal small ribonucleoprotein (snRNP) [35]. 复合物促进剪接体小核糖核蛋白(snRNP)的组装和功能[35]。snRNP comprises 由富含U-rich small nucleus RNA (snRNA) and 7 small (Sm) or Sm-like (LSm) proteins assembled into a complete stem ring structure in the cytoplasm [34][36]. This assembly process, which involves multiple 的小核RNA(snRNA)和7个小(Sm)或Sm样(LSm)蛋白组成,组装成细胞质中完整的茎环结构[34,36]。这种装配过程涉及多个SMN complex components, necessitates a cellular positional shift.复杂组件,需要蜂窝位置偏移。 Initially, 最初,snRNAs (excluding U6) are transcribed in the nucleus by RNA polymerase II as precursor RNAs, containing an additional stem-loop structure at the 3′ end and a monomethylated m(不包括U6)在细胞核中被RNA聚合酶II转录为前体RNA,在3'端包含一个额外的茎环结构,在7'端包含一个单甲基化的m7GpppG (m7G) cap (m5G)帽结构[37]。该前体structure at the 5′ end [37]. This precursor snRNA 随后通过其5′帽结构与包含多种蛋白质的subsequently binds to an snRNA export complex comprising multiple proteins through its 5′ cap structure, leading to its export into the cytoplasm [38].nRNA输出复合物结合,导致其输出到细胞质中[38]。 In the cytoplasm, 在细胞质中,Sm and Sm-like proteins first bind to the chloride conductance regulatory protein (pICln) and protein arginine methyltransferase 5 (PRMT5) complexes. These proteins are then prearranged into spatial positions and separated upon the addition of the SMN complex, which assembles the Sm and Sm-like proteins into protein binding sites on snRNAs, forming the complete snRNP with a seven-和Sm样蛋白首先与氯化物电导调节蛋白(pICln)和蛋白精氨酸甲基转移酶5(PRMT5)复合物结合。然后将这些蛋白质预先排列到空间位置,并在加入SMN复合物后分离,SMN复合物将Sm和Sm样蛋白组装成snRNA上的蛋白质结合位点,形成具有七元环结构的完整snRNP[39,40]。 最后,具有Smembered loop 核心的structure [39][40]. Finally, the snRNA with the Sm core undergoes methylation at its 5′ end, catalyzed by trimethylguanosine synthase 1 (TGS1), to form an m3G cap structure. This process is mediated by the SMN complex and importin β, facilitating the performance of splicing functions in the nucleus [15][41]. This assembly process is complex and involves different components playing unique roles. The focus of this section is a brief overview of the role of 在其5′末端经历甲基化,由三甲基鸟苷合酶1(TGS1)催化,形成m3G帽结构。该过程由SMN复合物和导入蛋白β介导,促进细胞核中剪接功能的表现[15,41]。这种组装过程很复杂,涉及不同的组件扮演独特的角色。本节的重点是简要概述DDX20 / Gemin3 in this process.在此过程中的作用。 First and foremost, the 首先,SMN complex serves a critical, nonreplaceable role in snRNP assembly, and DDX20/复合物在snRNP组装中起着至关重要的不可替代的作用,而DDX20 / Gemin3 is indispensable for the stabilization of the complex. Within the SMN complex, DDX20/对于复合物的稳定是必不可少的。在SMN复合物中,DDX20 / Gemin3 engages with SMN proteins, along with 与SMN蛋白以及Gemin2, 4, and 5. This interaction recruits 4和5结合。这种相互作用招募Gemin3 as a core component of the SMN complex, promoting its stability [42][43][44]. A sumoylation modification of the core components of the 作为SMN复合体的核心成分,促进其稳定性[42,43,44]。SMN complex, including 复合物的核心组分(包括Gemin3, is necessary for this interaction. A desumoylation modification of )的sumo-Gemin3 could decrease binding to several core proteins and potentially impact the cellular localization SMN complex [29]. As shown in Figure 的脱硫修饰可能会减少与几种核心蛋白的结合,并可能影响细胞定位SMN复合物[29]。如图2, 所示,Gemin3 forms an SMN complex with several other proteins and there is interaction between the various proteins.与其他几种蛋白质形成SMN复合物,并且各种蛋白质之间存在相互作用。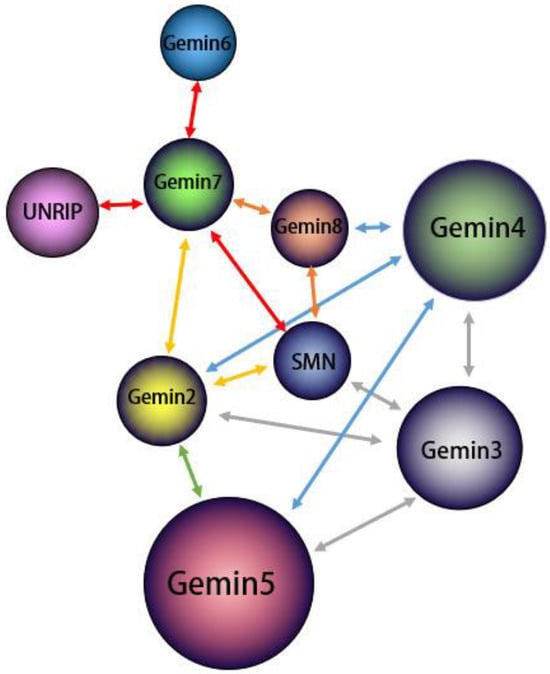
4. DDX20 Represses Transcription by Inhibiting Transcription Factors通过抑制转录因子抑制转录
In recent years, the role of 近年来,DDX20 in transcription has been increasingly reported and considerable evidence regarding its role as a transcription regulator has been reported. DDX20 primarily suppresses transcription by interacting with the nuclear receptor steroidogenic factor-1 (SF-1) [51]. 在转录中的作用被报道得越来越多,并且已经报道了大量关于其作为转录调节剂作用的证据。DDX20主要通过与核受体类固醇生成因子-1(SF-1)相互作用来抑制转录[51]。SF-1, a member of the transcription factor nuclear receptor superfamily, is a crucial regulator of the hypothalamic–pituitary–gonadal axis and the endocrine factor of the adrenal cortex [52]. 是转录因子核受体超家族的成员,是下丘脑-垂体-性腺轴和肾上腺皮质内分泌因子的关键调节因子[52]。DDX20 is highly expressed in steroid-producing tissues, which also highly express 在产生类固醇的组织中高表达,类固醇组织也高表达SF-1 [10]. [10]。DDX20 directly interacts with the 通过其非保守的C-terminal inhibitory domains of SF-1 via its nonconserved C-terminal domains to inhibit the transcriptional activity of SF-1 [20]. Further studies have elaborated on the specific mechanisms by which 端结构域直接与SF-1的C端抑制结构域相互作用,抑制SF-1的转录活性[20]。进一步的研究已经详细阐述了DDX20 interacts with 与SF-1 and inhibits its transcriptional activity. The transcriptional activity of SF-1, a transcription factor, is affected by other transcription factors, coregulatory factors, and post-translational modifications [53]. 相互作用并抑制其转录活性的具体机制。转录因子SF-1的转录活性受其他转录因子、共调控因子和翻译后修饰的影响[53]。Sumoylation, a ubiquitin-like modification that occurs following SF-1 translation, inhibits the ability of SF-1 to activate target gene expression [54]. zation是SF-1翻译后发生的泛素样修饰,可抑制SF-1激活靶基因表达的能力[54]。DDX20 enhances the sumoylation of 在与SF-1 mediated by protein inhibitor of activated STATs proteins (a type of 蛋白直接相互作用以抑制其转录活性后,增强由活化的STATs蛋白抑制剂(一种E3-SUMO ligase) after direct interaction with the SF-1 protein to inhibit its transcriptional activity [55]. This interaction also facilitates the relocalization of 连接酶)介导的SF-1的苏莫化[55]。这种相互作用也有助于SF-1 to discrete nucleosomes or lesions. Exploring this inhibitory mechanism revealed that Histone deacetylase is involved in sumoylation modification of transcription factor 重新定位到离散的核小体或病变。探索这种抑制机制表明,组蛋白去乙酰化酶参与转录因子SF-1 and interacts less with the corepressor; however, it may function as an E3 ligase during sumoylation [56].的sumo-ization修饰,并且与核心加压因子的相互作用较少;然而,它可能在苏莫酰化过程中作为E3连接酶起作用[56]。 SF-1 has a homologous gene in arthropods, known as 在节肢动物中具有同源基因,称为FTZ-F1 ((fushi tarazu factor-1) [57]. A study confirmed that 因子-1)[57]。一项研究证实,DDX20 interacts with 通过酵母双杂交测定法与副苜蓿中的FTZ-F1 in paralfalfa via a yeast two-hybrid assay and reported that DDX20 can suppress 相互作用,并报道DDX20可以通过类似于RNAi抑制SF-1的机制抑制FTZ-F1 expression via a mechanism similar to that of S表达[58]。F-1 inhibition by RNAi [58]. FTZ-F1 is closely related to vitellogenin (与卵黄原素(VTG) and is involved in the development of vitelline in the ovary and can influence the secretion of several endocrine hormones [59]. The inhibition of )密切相关,参与卵巢中卵黄素的发育,可影响几种内分泌激素的分泌[59]。通过DDX1抑制FTZ-F1 via DDX20 eventually affects ovarian development.最终会影响卵巢发育。 The 叉头转录因子(Forkhead transcription factor (FOXL) 2, a transcription factor closely related to OXL)2是与FTZ-F1 and DDX20, also plays a significant role in regulating ovarian development. In the ovary, FOXL2 is involved in regulating cholesterol and steroid metabolism, apoptosis, reactive oxygen species detoxification, and cell proliferation [60]. Initially, it was discovered that 和DDX20密切相关的转录因子,在调节卵巢发育方面也起着重要作用。在卵巢中,FOXL2参与调节胆固醇和类固醇代谢、细胞凋亡、活性氧解毒和细胞增殖[60]。最初,发现DDX20 interacted with 与FOXL2 and their coexpression in cells enhanced FOXL2-mediated ovarian cell death [61]. Subsequent studies confirmed that 相互作用,它们在细胞中的共表达增强了FOXL2介导的卵巢细胞死亡[61]。随后的研究证实,FOXL2 interacts with 与DDX20 and 和FTZ-F1 and not only downregulates VTG synthesis by regulating follicular cell apoptosis through DDX20 but also potentially regulates the steroidogenic pathway through 相互作用,不仅通过DDX20调节滤泡细胞凋亡来下调VTG合成,而且还可能通过FTZ-F1.调节类固醇生成途径。 In addition to 除F-1, DDX20 has been discovered to interact with two other transcription factors to inhibit their activity by different mechanisms compared with that used with SF-1. First, DDX20 can engage with the mitotic Ets transcription inhibitor (PE-1/METS) via its C-terminal domain. Ets is a transcription factor that serves as a nuclear target to activate the Ras–MAPK signaling pathway, while 外,已发现DDX20与另外两种转录因子相互作用,与SF-1相比,通过不同的机制抑制其活性。首先,DDX20可以通过其C端结构域与有丝分裂的Ets转录抑制剂(PE-1 / METS)结合。Ets是一种转录因子,可作为核靶点激活Ras-MAPK信号通路,而Ets家族的另一个成员PE-1/METS, another member of the Ets family, acts as an Ets inhibitor to repress the Ras-dependent proliferation of Ets target genes, thus causing macrophage growth arrest [62].则作为Ets抑制剂抑制Ras依赖性Ets靶基因增殖,从而导致巨噬细胞生长停滞[62]。 DDX20 interacts with the N-terminal domain of 通过其C端结构域与PE-1/METS via its C-terminal domain, which has an inhibitory effect. In this process, DDX20 also recruits factors such as histone deacetylase 的N端结构域相互作用,具有抑制作用。在此过程中,DDX20还募集组蛋白去乙酰化酶HDAC-2 and HDAC5 [63]. However, interestingly, this transcriptional inhibition mediated by 和HDAC5等因子[63]。然而,有趣的是,这种由DDX20 targets only individual promoters, su介导的转录抑制仅针对单个启动子,例如ch as c-myc and cdc2, without affecting Ets ternary complex-facilitated transcription [64].-myc和cdc2,而不影响Ets三元复合物促进的转录[64]。 Thus, DDX20 does not undergo sumoacylation as DDX5 and its transcription regulation activity do not solely rely on a single intrinsic function but involve multiple mechanisms, many of which depend on its unique noncore C-terminal domain. This multifaceted approach to transcriptional regulation reinforces the complexity of the function of DDX20 in this essential cellular process.5. Biogenesis of DDX20 and miRNA
DDX20/Gemin3 also contributes to the maturation of miRNA. The biogenesis of miRNA begins with the transcription of miRNA genes into primary miRNA (pri-miRNA) by RNA polymerase II. The pri-miRNA is subsequently processed into a precursor miRNA (pre-miRNA) of ~70 nucleotides by a complex containing the RNase III endonuclease Drosha and a protein containing a double-stranded RNA-binding structural domain (dsRBD) named Pasha (DGCR8) [65][66][77,78]. Subsequently, the stem–loop-structured pre-miRNAs are transported into the cytoplasm, which is dependent on the GTP-dependent transport protein Exportin-5 (Exp5) [67][79]. In the cytoplasm, the RNase III endonuclease Dicer processes pre-miRNA into an siRNA–miRNA duplex of ~22 nucleotides. The mature miRNA strand is subsequently retained in the RNA-induced silencing complex (RISC) along with other proteins [68][80]. Although most Gemin3 and Gemin4 are components of the SMN complex, the complexes of Gemin3 and Gemin4 isolated from HeLa and neuronal cells are not part of the SMN complex. Instead, these complexes coprecipitate with polyribosomes [69][70][71][71,81,82]. Numerous studies have established the connection of Gemin3/DP103 and Gemin4 with the Argonaute (Ago) protein family member Argonaute [60][72][73][60,83,84]. Figure 4 shows the mIRNAde1 maturation process and the role Gemin3 plays in it.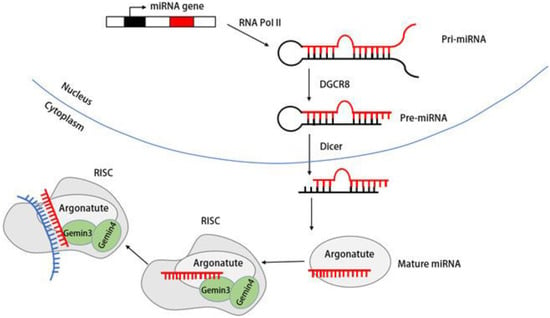
6. Functions of DDX20 in the Innate Immune Signaling Pathway
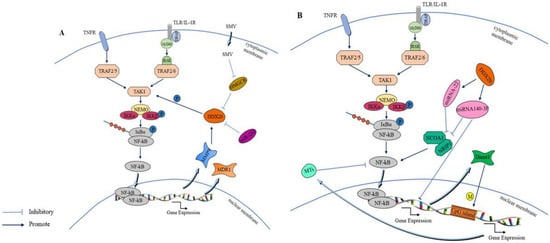
6.1. Effect of DDX20 on the NF-κB Signaling Pathway
The NF-κB family comprises six distinct components. When activated, they produce various proinflammatory factors that regulate inflammation and significantly contribute to innate immunity [77][78][87,88]. The NF-κB signaling pathway controls the expression of proinflammatory cytokines and anti-infective factors, including TNF-α, interleukin-1 (IL-1), IL-6, IL-8, adhesion molecules, and cc chemokine ligand 5 (CCL5) [79][89]. In addition, NF-κB signaling pathway governs cellular processes such as cell proliferation, differentiation, and apoptosis [80][81][90,91]. While the NF-κB signaling pathway has an antiapoptotic role, its dysregulation has been implicated in the pathogenesis of most human malignancies [82][92]. Therefore, manipulating the NF-κB signaling pathway can provide a path for developing novel methods to combat diseases such as cancer [83][93]. For instance, alterations in DDX20 expression in cancer tissues can affect NF-κB activity, leading to cancer development [84][94]. DDX20 can have two distinct effects on the NF-κB signaling pathway. Numerous studies have reported that DDX20 does not directly affect NF-κB activity but rather acts through a naturally occurring small non-coding RNA (miRNA) intermediate. The present study demonstrated that DDX20 can modulate the signaling of the NF-κB pathway through miRNA-22, miRNA-140-3p, and miR-222 [8][85][8,95]. Additionally, miR-361-5p was found to regulate DDX20, thereby influencing NF-κB pathway signaling [13]. First, DDX20 can inhibit NF-κB activity. DDX20 and miRNAs together form a ribonucleoprotein complex, and miRNA library screening has revealed that several miRNAs can inhibit NF-κB activation. This inhibition prevents the activation of the NF-κB signaling pathway [8]. It has been well established that miRNAs can inhibit NF-κB activity by regulating the expression of two NF-κB coactivators, namely nuclear receptor coactivator protein 1 (NCOA1) and nuclear receptor-interacting protein 1 (NRIP1) [86][96]. Conversely, DDX20 can also enhance NF-κB activity, notably by impairing miRNA function by decreasing its own expression, leading to impaired NF-κB inhibitory miRNA function [8][87][8,97]. Another way DDX20 can inhibit the NF-κB signaling pathway is through miRNA-140 dysfunction, which increases expression of its downstream target gene Dnmt1 and the methylation of CpG islands in the promoter region of metallothionein (MTs) and decreases MT expression, leading to enhanced NF-κB activity [9]. Second, DDX20 can boost the NF-κB signaling pathway by enhancing the phosphorylation of TAK 1, where it acts as a cofactor of TAK1, thus enhancing the activity of the NF-κB signaling pathway [88][98]. More details regarding this aspect will be discussed in the subsequent sections.6.2. DDX20 Affects p53 Signaling Pathway Conduction
The TP53 gene encodes the key p53 transcription factor and evolutionarily conserved tumor suppressor involved in maintaining genomic stability [89][99]. To accomplish this, it activates DNA repair responses and initiates apoptosis in damaged host cells [90][100]. Its activation controls core programs such as cell cycle arrest and apoptosis [91][101]. In addition, p53 is closely related to immune responses as well as various inflammatory diseases [92][102]. In fact, the effect of DDX20 on the p53 signaling pathway can be realized by directly affecting the pivotal p53 protein. Changes in DDX20 expression can consequently impact the organism’s state through the p53 signaling pathway. Reportedly, DDX20 interacts with p53 protein through its C-terminal structural domain [1]. The normal expression of DDX20 stabilizes motor neurons and uses genomic stability and regulates Mdm2 splicing to restrain the p53 signaling pathway, thereby preserving normal neural development [4]. In this context, several cytokines, such as oligodendrocyte transcription factor 2 (Olig2) and Epstein–Barr (EB) nuclear antigen 3C (EBNA3C), can directly interact with DDX20 to stabilize its expression. This interaction inhibits transcription and apoptosis caused by the p53 signaling pathway and its downstream genes within the organism [4][93][4,67].7. DDX20 Plays Different Roles in Cancers through the NF-κB Signaling Pathway
DDX20 not only plays a role in the invasion of multiple pathogens but also plays an active role in the case of multiple tumorigenesis. In breast cancer, DDX20 is involved in cell signaling pathway activity, which is a key factor in tumorigenesis. The NF-κB signaling pathway is more closely linked to tumor development, and, reportedly, it can improve cancer cell survival, promote cancer cell angiogenesis and migration, and has other characteristics alongside being associated with the poor prognosis of cancer diseases [94][106]. It is because of the role of the NF-κB signaling pathway in tumor growth that it can be used to inhibit carcinogenesis [95][107]. DDX20 is an important cofactor for the phosphorylation of transforming growth factor-β-activated kinase-1 (TAK1) by NF-κB-activated IκB kinase 2 (IKK2), enhancing the activity of the NF-κB signaling pathway by stimulating TAK1 phosphorylation [96][108]. TAK1 is a member of the mitogen-activated protein kinase (MAPK) family that plays a key regulatory role as an upstream component of the NF-κB signaling pathway [97][109]. Enhanced NF-κB signaling pathway activation increases in the expression of two downstream products, matrix metalloproteinase 9 (MMP9) and multidrug resistance gene 1 (MDR1), and, as the chief role of MMP9 is to degrade the extracellular matrix, this change further increases tumor metastasis and drug resistance [19][98][19,110]. Conversely, enhanced NF-κB activity and increased downstream MMP9 expression would also lead to increased DDX20 expression. Thus, the establishment of the DDX20–NF-κB–MMP9 axis could better reveal the mechanism by which DDX20 can promote cancer development [99][69]. In addition, DDX20 may exhibit an miRNA-processing role in breast cancer. A group of studies reported that DDX20 exhibited a negative correlation with an miRNA, namely miR-222, suggesting that DDX20 affects the NF-κB activity through miR-222 to promote breast cancer development [85][95]. Based on this property of DDX20 in breast cancer, researchers believe that DDX20 can serve as an active alternative to certain anticancer drugs. DDX20 enhances the sumolylation modification of YAP, thereby increasing YP-TEAD dependence and statin sensitivity in patients with triple-negative breast cancer [16]. Statins, such as simvastatin (SMV), are cholesterol-reducing lipophilic statins that inhibit DDX20 expression by inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), which is positively associated with DDX20 [100][103]. Figure 5A demonstrates that DDX20 affects tumorigenesis and development through the NF-κB signaling pathway. Further studies have reported that simvastatin downregulates DDX20 not only through the classical mevaleric acid pathway and the downstream component of RHoA but also through the miRNA-mediated nonclassical pathway [101][102][111,112]. Simvastatin ultimately inhibits breast cancer by decreasing DDX20 expression. In addition to its role through the NF-κB signaling pathway and miR-222, DDX20 acts through the cellular redox pathway and Wingless/Integrated(Wnt) signaling pathway. In cancer stem cells (CSCS), DDX20 drives a positive feedback loop wherein DDX20 promotes its transcriptional regulation via transcription factor 4 (TCF 4) to stimulates the aggressiveness of breast cancer via Wnt/beta-catenin signaling [103][113]. Another example of the carcinogenic effect of DDX20 is in prostate cancer. High DDX20 expression in the tumor tissue of patients with prostate cancer also enhances tumor growth and metastasis via the DDX20–NF-κB–MMP9 axis [11]. These results suggest a role of DDX20 in tumor metastasis.