Initially, and still in use in most of the products that are currently applied in the clinic, bioceramic materials have been synthesized from precursor salts using traditional industrial processes that required high temperatures, followed by the casting of bulk implants or the quenching of powders. However, since the early 1990s, research has begun on bioactive ceramics using an alternative process, the sol–gel technique
[21][22][28,29]. The synthesis of nanomaterials can be broadly classified into two approaches: “top-down” and “bottom up”. Top-down synthesis involves the deconstruction of larger materials to produce nanostructures. Bottom-up synthesis constructs nanomaterials from basic building blocks like atoms and molecules. The sol–gel technique is an example of a bottom-up approach for producing bioceramics from small molecules. The method consists of several stages involving chemical and physical processes. The chemical process begins with the reaction of precursor monomers to form oligomers in solution (sol), which in turn polymerize into a network (gel) in the form of an integrated network of discrete particles or network polymers
[23][30]. In general, the mechanism of hydrolysis of the precursor monomers and their condensation to oligomers are the most critical steps in sol–gel chemical synthesis. These mechanisms determine the structure and composition of the resulting material. The synthesis parameters that bias the structure toward linear or branched structures are also critical issues, which play a crucial role in determining the properties and performance of the final material.
A major advantage of the sol–gel method is that it is possible to obtain materials with high purity and homogeneity at the molecular scale and to control the surfaces, interfaces and porosity of the materials obtained at the nanometric scale
[24][31]. The sol–gel method produces homogeneous sols that can be converted into gels with a very high volume of nanopores. This nanoporosity is undoubtedly one of the most important characteristics of advanced bioceramics, as it translates into a higher specific surface area, greater reactivity and, therefore, faster kinetics in the bioactive response
[25][32]. The sol–gel method can be used to synthesize bioceramics with different chemical compositions, which enables the production of a wide range of materials. Besides, it is a cost-effective technique, as it requires lower reaction temperatures and simpler equipment than other high temperature burning and thermal industrial processes. The low reaction temperature reduces the energy consumption and gas emissions, which contribute to its environmental sustainability.
On the other hand, control of the processing method makes it possible to vary the morphology of the synthesized materials and, thus, obtain particles, films, monoliths or fibers
[26][27][33,34]. Additionally, the whole process can be easily scaled up for large-scale production. All these characteristics make it possible to obtain bioceramics with high added value, and there is much interest in finding new synthesis routes and processes that align these advantages with other equally important commercial aspects, with respect to the economic viability of scaling up sol–gel production
[24][31].
There are a large number of bioceramic materials on the market that are used in dentistry to stimulate the repair and regeneration of dental tissues, such as enamel, dentin and pulp, as well as bone defects in oral and maxillofacial surgeries. Most of them correspond to ceramic products obtained by conventional methods, such as melting in the case of vitreous materials or high temperature heat treatment of precursor salts. There are, however, a few commercially available products, such as NovaBone or NanoBone, that have begun to incorporate advances in sol–gel synthesis, giving them new textural properties in terms of surface and porosity.
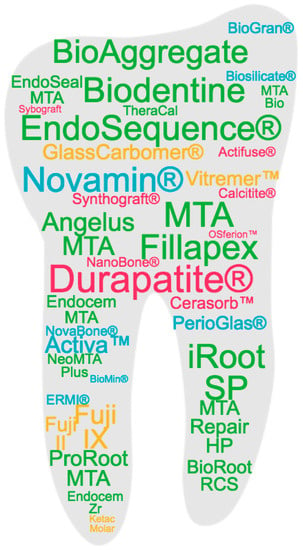
Figure 1 represents the commercial products based on bioceramics in clinical use that are most studied in the literature, marked in different colors according to the main type of bioceramic material component, namely bioactive glass (BG), calcium phosphate (CaP) or calcium silicate (CaSi), as detailed in the following sections.
Figure 1. Commercial products based on bioceramics in clinical use that are most studied in the literature. Based on a search of the Scopus database, the products are represented with a font size according to their frequency in the title of an article and with a color code corresponding to the different types of bioceramics classified in the work: blue for bioactive glass (BG); yellow for glass ionomeric; pink for calcium phosphate (CaP); green for calcium silicate (CaSi).
2. Bioactive Glasses
Bioactive glasses (BGs) were discovered by Hench in 1969
[28][35]. His team at the University of Florida found that these materials elicited a biological response when they came into contact with the physiological environment, which led to a new approach for the application of biomaterials in clinical practice
[29][30][31][36,37,38]. The original bioactive glass composition, 45S5, formulated on weight bases from 45% SiO
2, 24.5% Na
2O, 24.5% CaO and 6% P
2O
5, was commercially trademarked as Bioglass
® [28][35] and many of the commercial products still available use this composition. Variations in this formulation, including other compounds such as K
2O, MgO, CaF
2 and B
2O
3, have been implemented and have shown altered properties such as dissolution rates and bioactivity. In addition to variations in their composition, different processing methods have also been reformed, such as their manufacture in the form of implant-like monoliths, granules or particles, pastes and cements. An excellent recent paper reviews all of the commercial BGs devices approved for therapeutic application, including hard tissue scaffolding, dental remineralization, soft tissue repair and cancer treatment
[32][39].
In the field of dentistry, Endosseous Ridge Maintenance Implant (ERMI
®), PerioGlas
®, BioGran
®, NovaBone
® and NovaMin
® are commercialized bioactive glass products based on the 45S5 composition. ERMI
® is used in the form of a monolith to be implanted into the void left following tooth extraction to encourage bone formation and to provide a stable ridge for future tooth replacement
[33][34][35][40,41,42]. PerioGlas
® is used for the repair of periodontal and smaller oral defects. It was the first product to be delivered as glass powder, ranging from 90 to 710 μm, which makes surgery easier by allowing the operator to pack the wound with powder rather than fit a premade product into the void
[36][37][38][43,44,45]. BioGran
® has a similar application to PerioGlas
®, but with a narrow particle size of 300 to 360 μm
[39][40][41][42][46,47,48,49]. NovaBone
® is available in the form of dental putty combined with a binder to improve handling for grafting, and also as interconnected porous granules for faster bone integration and remodeling for reconstructive surgeries, such as ridge maintenance and augmentation, extraction sites, implant preparation and placement
[43][44][45][50,51,52]. NovaMin
® is applied to toothpaste for treating tooth hypersensitivity. It has a fine particle size with a D50 of 18 μm, which allows the glass to have a higher probability of entering the dentin tubules in the teeth
[46][47][48][49][50][51][53,54,55,56,57,58]. Besides, NovaMin
® is used in polishing and teeth whitening procedures to stimulate mineralization
[26][33] and has been shown to help to treat gingivitis
[52][59]. BioMin
® is a modification of the 45S5 composition containing either fluorine (BioMin F) or chlorine (BioMin C) to aid in apatite precipitation for dentin hypersensitivity
[53][54][60,61]. Another glass composition variation (wt.%), 48.5% SiO
2, 23.75% Na
2O, 23.75% CaO and 4% P
2O
5, is used to produce the Biosilicate
® glass ceramic. Biosilicate, engineered under a controlled double-stage heat treatment, is effective in the clinical reduction of sensitivity in enamel and dentine
[55][56][57][62,63,64].
Bioactive glasses of undisclosed exact composition are also marketed as components in composite formulations with resins, polymers and other agents for use as restorative esthetic composites and biomaterials for endodontics. Activa™ BioACTIVE contains a shock-absorbing component, making it resistant to fracture and wear. It chemically bonds to the tooth and releases and recharges calcium, phosphate and fluoride ions, providing long-term benefits
[58][59][65,66]. GuttaFlow
® is composed of gutta-percha, polydimethylsiloxane, platinum catalyzer, zirconium dioxide and BG, showing low solubility, low porosity, alkalization capacity, dentin penetrability and cytocompatibility
[60][67]. A newly developed bioactive glass-based cement, Nishita Canal Sealer BG (NCS-BG), is now being commercially marketed as a root canal sealer and applied within clinical endodontic treatments
[61][68].
Finally, Ting et al.
[62][69] reported that the 58S glass (nominal composition 60 mol% SiO
2, 36 mol% CaO and 4 mol% P
2O
5) was one of the first sol–gel-derived bioactive glass compositions developed and commercialized by NovaBone Products LLC (Alachua, FL, USA), although hydroxyapatite (HA) was found to form within the 58S glass during sol–gel synthesis after thermal stabilization, where it was heated to 700 °C.
Although with a distinctive bioactive ability in restorative dentistry, glass-ionomer materials also deserve a separate mention. They are a group of materials composed of silicate glass powder and an aqueous solution containing polyacrylic acid that solidifies due to an acid–base reaction
[63][21]. Glass-ionomer cements (GICs) are considered bioactive because they release biologically active ions, such as fluoride, calcium, strontium, sodium, phosphate or silicon, that result in long-term durable bonds at the tooth–restoration interface
[64][65][70,71]. Commercial GICs, Fuji IX
[66][67][68][69][72,73,74,75], Ketac Molar
[70][71][76,77], Glass Carbomer
® [72][73][74][75][78,79,80,81], have been shown to promote remineralization in the mouth. Resin-modified products, Fuji II
[76][77][78][82,83,84] and Vitremer™
[79][80][81][85,86,87], also contain ion-leachable glass powder, as well as the water-soluble organic monomer 2-hydroxyethyl methacrylate (HEMA), and are widely used as alternatives to amalgam.
3. Calcium Phosphates
Synthetic calcium ortho-phosphate (CaP) materials can be prepared with a chemical composition very similar to that of the inorganic part of human bones and teeth. They are widely used in medicine for their biocompatibility, bioactivity and osteoconductivity properties
[82][88]. Bone and dentine contain about 70% calcium phosphate (CaP) mineral in the form of a poorly crystalline, highly substitute apatite phase, consisting of very small crystallites, with a thickness of only 5 nm. Enamel, on the other hand, consists almost exclusively of hydroxyapatite prisms up to 100 µm in length and oriented in structures that confer resistance to abrasion
[83][24]. Several dental specialties deal with the invasion into or the treatment of the surrounding bones, such as the filling and/or reconstruction of a traumatic or degenerative multi-walled bone defect, augmentation of the sinus floor, augmentation of alveolar ridges, the filling of periodontal or other alveolar bone defects, tooth sockets, osteotomies and the preservation of the alveolus for the preparation of an implant site. Depending on the application, different compounds, such as monocalcium phosphate (MCP; Ca(H
2PO
4)
2), dicalcium phosphate (DCPA; CaHPO
4), tricalcium phosphate (TCP; Ca
3(PO
4)
2) or hydroxyapatite (HA; Ca
10(PO
4)
6(OH)
2), as well as their processing in different formats, such as particles, granulates, dense blocks, porous parts, pastes or coatings, have been developed. Research on this type of material is very extensive, as some recent reviews in the bibliography show
[84][85][86][89,90,91]. Dorozhkin
[84][89] highlights that the first publications on the application of CaPs in dentistry deal with their inclusion in toothpaste formulations to promote remineralization and reduce tooth sensitivity, and reviews these materials according to two types of classification, namely the CaP compound formulation and the specific application for the different specialties in dentistry
[84][89].
The first reported commercial CaP products are based on β-TCP and HA, such as Synthograft
® (β-TCP)
[87][88][89][90][92,93,94,95], Durapatite
® (HA)
[91][92][93][94][96,97,98,99], Calcitite
® (HA)
[95][96][100,101] and Alveograft
® (HA)
[97][102]. Also, β-TCP based products are subsequent to Cerasorb™
[98][99][100][103,104,105] and OSferion™
[101][106]. Actifuse
® is a porous silicate-substituted calcium phosphate
[102][103][107,108]. Synthetic nano-crystalline HA is commercialized as a single component bone graft by Sybograft
® [104][109], and as a composite formulation NanoBone
® consisting of nanocrystalline HA embedded in a silica gel matrix, produced using a sol–gel process
[105][106][107][110,111,112]. Besides, CaPs are incorporated as components in self-setting products, such as Endo Sequence
® BD Sealer, a premixed ready-to-use injectable cement for sealing applications, which contains MCP
[84][108][89,113].
4. Calcium Silicates
Calcium silicates, mainly Ca
3SiO
5 and Ca
2SiO
4, are the basic compounds in bioactive endodontic cements (BECs)
[109][110][26,114]. BECs are bioceramics widely used in endodontics as restorative cements used in vital pulp therapy and endodontic sealers, due to their high biocompatibility, intrinsic osteoconductive activity and ability to induce regenerative responses as dentin bridges that promote better sealing of the pulp-capped site
[59][111][66,115]. These calcium silicates compounds are capable of reacting with water at a physiological temperature, causing a hydraulic setting reaction. Originally the first product formulation was described as a powder composed of calcia, silica and alumina oxides and was then named mineral trioxide aggregate (MTA), which is still a generic name used for BECs in dentistry
[112][116]. In fact, MTA is based on Portland cement, which was revisited by Torabinejad et al.
[113][117] for its use in endodontics. Despite its excellent properties, some problems in its clinical application, such as the long setting time, tooth discoloration, high cost and difficult handling, have driven the development of new formulations.
The first clinically approved formulation was ProRoot MTA
[114][118]. The initial setting time has been reported from 70 to 74 min
[110][114]. In 2002, the gray ProRoot MTA (GMTA) was substituted by the new white ProRoot MTA (WMTA), free from tetracalcium aluminoferrite to reduce the problems concerning tooth discoloration
[115][119]. MTA Angelus
[116][117][120,121] followed with a similar composition based on Portland cement, but without the calcium sulphate dehydrate (gypsum). Further products marketed with shorter setting times are Biodentine
[118][119][120][121][122][123][124][125][122,123,124,125,126,127,128,129], Endocem MTA
[126][127][128][129][130,131,132,133], MTA Bio
[130][131][134,135], EndoSeal MTA
[132][133][136,137] and MTA Fillapex
[134][135][136][137][138][139][138,139,140,141,142,143]. A setting time of as little as 0.3 min has been reported for TheraCal
[140][141][142][143][144][144,145,146,147,148] because of the use of resin and light cure technology. The radiopacifying agent used is another important element that has been studied for the improvement of these products. ProRoot MTA contains about 2 at.% Bi
[115][119], which may not only produce tooth discoloration but also reduce its biocompatibility
[145][149]. As an alternative to bismuth oxide, other compounds have been used, such as tantalum oxide Ta
2O
5, which is used in BioAggregate
[146][147][148][149][150][150,151,152,153,154] and NeoMTA Plus
[109][151][152][153][26,155,156,157]. ZrO
2 is another agent widely used in products such as Endocem Zr
[127][154][131,158], EndoSequence
[155][156][159,160], iRoot SP
[157][158][159][160][161,162,163,164], BioRoot RCS
[161][162][163][164][165,166,167,168] and the previously mentioned Biodentine. MTA Repair HP, notable for its low setting time and fast bioactive response in vitro
[165][25], contains CaWO
4 as a radiopacifying agent and consists of tricalcium silicate nanoparticles with high aspect ratio, which provide to the precursor material an elevated surface area to maximize the hydration reaction
[166][167][169,170].