You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Anna Paula Azevedo de Carvalho.
Significant amounts of fermented food waste are generated worldwide, promoting an abundance of residual biomass that can be used as raw material to extract bioactive peptides, fermentable sugars, polyphenols, and valuable compounds for synthesizing bioproducts. Therefore, generating these high-value-added products reduces the environmental impact caused by waste disposal and increases the industrial economic value of the final products. The fermentation process favors the synthesis of products with high added value. However, the use of fermentation also leads to the production of fermentative residues containing metabolites, extracellular enzymes, and other byproducts generated by microbiological metabolism.
- fermentation
- bioactive compounds
- bioeconomy
- biomass residues
- winery wastes
1. Animal Feeding
Very recently, phenolic compounds from rich-grape marc (or pomace) from the País winery, an abundant resource suggested as having a possible in animal feed, were considered for potential positive environmental effects on enteric CH4 emissions in Chile by Suescupun-Ospina et al. (2023) [85][1]. The authors evaluated the effect of the oven- and freeze-drying process and showed that oven-dried grape marc had the highest proanthocyanidin content and lower CH4 production [83][2].
2. Biofuels
The production of cleaner fuels can be accomplished by improving the refining of environmentally friendly technologies and by adding synthetic fuels or ethanol [48][3]. Within environmentally friendly technologies, we use residual biomass to synthesize bioethanol through the fermentation process of substrates with a high carbohydrate content for degradation. The use of sugarcane juice to synthesize bioethanol is already a reality. However, this substrate competes with the food industry [144][4]. To resolve this problem, other biomass resources, such as byproducts and wastes from fermented food factories, stand out as substrates since they are also rich in the carbohydrates needed for fermentation [77,79,97][5][6][7].
The cellulose and hemicellulose in lignocellulosic biomass can be chemically or enzymatically degraded, generating hydrolyzed sugars that provide raw materials for microorganisms to act upon during fermentation [145][8]. Lignin, the third component, is formed by aromatic polymers, and its catabolism generates a mixture of aromatic components that can be enzymatically converted into a set of organic molecules in the form of D-3,2-butanediol [79[6][9],143], methane [64][10], poly(3-hydroxybutyrate (PHB) [97][7] and PHA [69][11], and tartrate or tartaric acid [97][7].
Unlike bread waste, which is rich in fermentable sugars for H2, ethanol, methane, and recovery, some biomass residues, such as wood bark, are not easily converted into biofuels through the application of microorganisms. The use of the gasification process with these residues produces a synthesis gas (syngas) that offers a solution to this problem, such as the conversion of CO and H2 (which are present in the syngas) into multicarbon compounds [146][12]. Such an approach may be extended to other fermented biomass residues.
The production of fuels covers the use of various residues, both from agriculture and the food industry. Food waste occurs in large quantities worldwide; thus, anaerobic digestion can be employed, including H2 in the fermentation stage [76][13] and CH4 in the methanogenesis stage [77][5]. This process, also called dark fermentation, uses an environmentally friendly approach to solve the problem of food waste and delivery residue to synthesize industrial products. Several microorganisms can be used, including hydrolytic, acidogenic, and acetogenic bacteria, to form VACs, metabolites, CO2, CH4 [64][10] alcohols [77][5], and H2 [76][13]. The use of residues for H2 synthesis is still being optimized for use on an industrial scale, which brings other possibilities for study and evaluation regarding the topic [147,148][14][15].
Moreover, it has already been proven that dual-stage H2 production achieves 20% more biogas than single-stage production [149][16]. In the methanogenic phase, the VACs thus produced are transformed into CH4 and are recovered as biofuel, whereby part of the methane remains dissolved and can be treated for application in biofertilizers [150][17]. Dark fermentation encompasses the use of waste to synthesize environmentally friendly products for industrial commercialization. Thus, its optimization is essential for food and agro-industrial waste re-usage [151][18]. Figure 41 exemplifies the synthesis steps of some biofuels via dark fermentation.
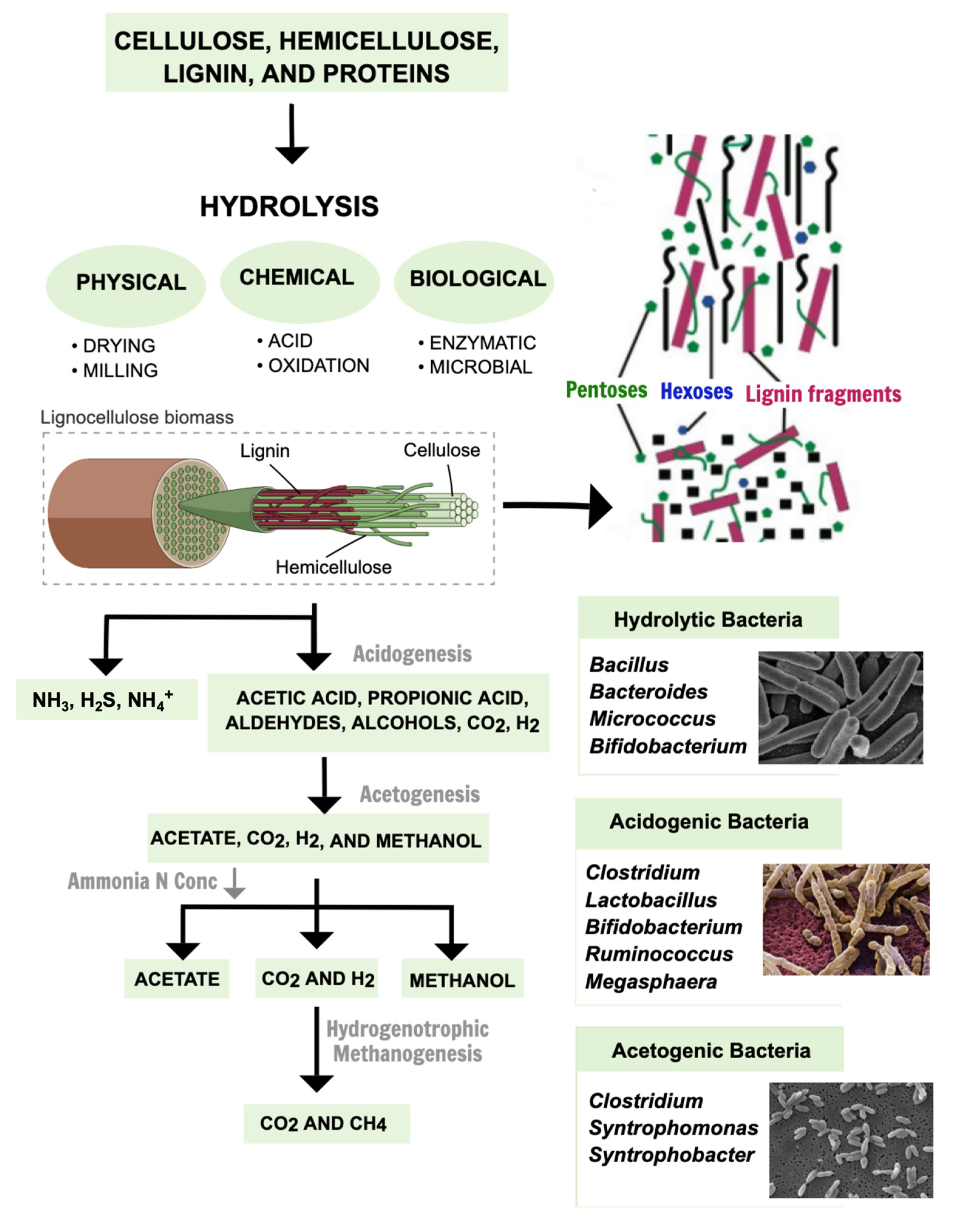
Figure 41.
Synthesis steps for some of the biofuels obtained via dark fermentation.
To produce a range of compounds such as acetate, ethanol, butyrate, and butanol, fermentation by microorganisms depends on acetyl coenzyme A (acetyl-CoA). The oxidation of H2 to 2H+ with H2O, or of CO with H2O to 2H+, provides the reducing equivalents for the reduction of CO2 to form methanoic acid (HCOOH), from methylene tetrahydrofolate (CH-THF) to methenyl tetrahydrofolate (CH2-THF), from CH2-THF to methyltetrahydrofolate (CH3-THF), and from CO2 to CO. Acetyl-CoA synthase/CO dehydrogenase catalyzes the formation of acetyl-CoA from methyl group bonding, CO group bonding, and coenzyme A (CoA). The metabolic pathways of acetyl-CoA reduction, which are involved in the fermentation of biomass conversion into a range of biofuel compounds, are displayed in Figure 52 [151,152,153][18][19][20].
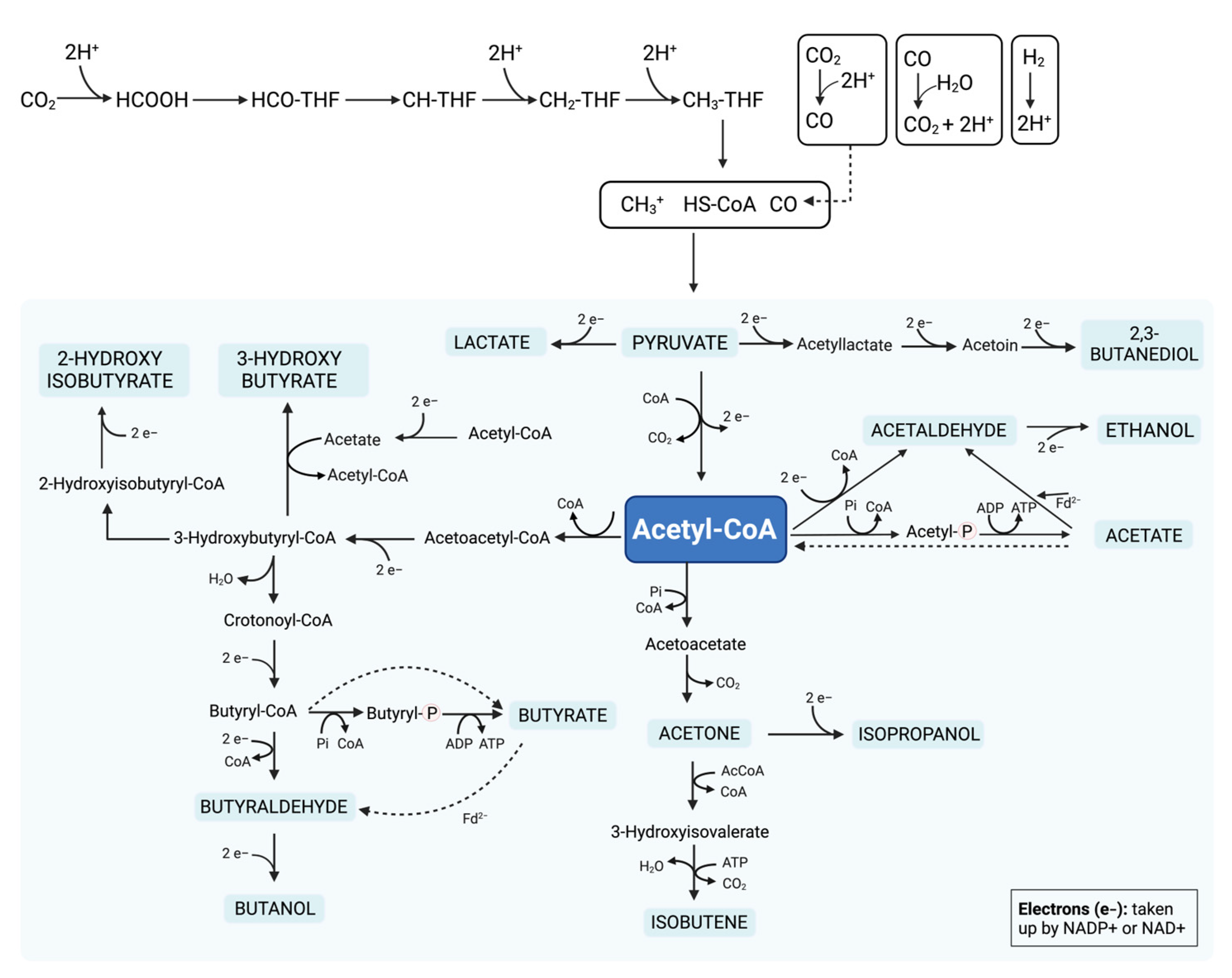
Figure 52.
Metabolic pathways of acetyl coenzyme A (acetyl-CoA) reduction, involving the fermentation of biomass conversion into a range of biofuel compounds.
Hydrogenogenic carboxydotrophic microorganisms (HCM) conserve energy by synthesizing H2, in which a monofunctional CO dehydrogenase oxidizes CO. The electrons from the oxidation process undergo the reduction of protons in hydrogen molecules through an energy-converting hydrogenase (ECH). Furthermore, ECH couples H2 to the membrane translocation of protons/sodium ions, which generates an ion gradient, driving ATP synthesis. HCM use is independent of the acetyl-CoA pathway [151,152,153][18][19][20].
Bread and bakery product wastes have an interesting composition (up to 70% of carbohydrates, mainly starch), which is attractive as a nutrient source for microorganisms and can improve the circular economy by reusing energy-rich food wastes for gas production [77][5] and energy recovery [76][13]. Bread waste was employed to produce organic acids by lactic fermentation (Lactobacillus amylovorus DSM 20532) and was sequentially employed for hydrogen (H2) production (3.1 mol H2 mol−1 glucose) and energy recovery (54 MJ t−1 dry waste) by photo-fermentation (Rhodoseudomonas palustris 42OL) [76][13]. Using biorefinery concepts and a circular economic approach, bread waste was employed to produce bioethanol [77][5], butanediol [78[6][21],79], and biomethane [77][5]. The fermentable sugars from out-of-date bread supplied by local supermarkets in the United Kingdom were first submitted to acid/enzymatic saccharification to recover glucose-rich hydrolysates, which were sequentially fermented with Saccharomyces cerevisiae KL17 to produce ethanol with higher efficiency, using the fed-batch mode of cultivation [77][5]. In further experiments, Narisetty et al. (2022) [77][5] employed solid residues (from acid/enzymatic hydrolysis, along with the respective SSF residues after ethanol production) under anaerobic digestion to yield biomethane, revealing a biochemical methanation potential of up to 379 mL CH4/g. Likewise, other studies have previously reported glucose-rich enzymatic hydrolysate from bread waste being submitted for fermentative production by Bacillus amyloliquefaciens [78][21] and by Enterobacter ludwigii [79][6] to accumulate D-2,3-butanediol.
3. Biopolymers and Bioplastics
The continuous production of plastics from the petrochemical industry has generated an exorbitant amount of waste for disposal. Such waste is part of approximately 140 million tons of plastics produced in 2022, and an estimate of plastic waste in the ocean has reached a level of 150 million tons [154,155][22][23]. Figure 63 shows a forecast for global plastics generation from 2002 to 2050 [156,157][24][25].
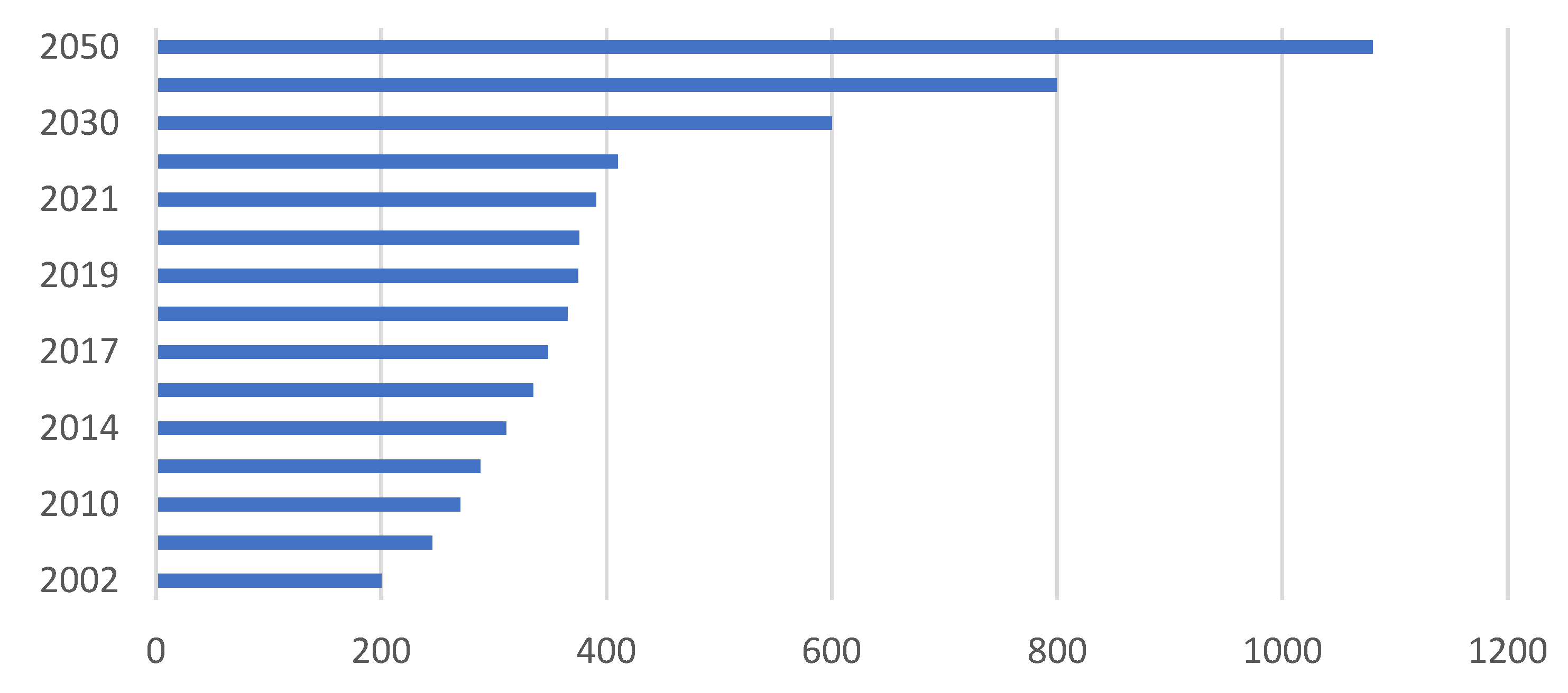
Figure 63.
Forecast of plastics use generated worldwide from 2002 to 2050.
This challenging scenario has motivated researchers to evaluate biodegradable plastics produced by the fermentation of microorganisms to replace petroleum-based plastics [158,159,160,161][26][27][28][29]. The production of biopolymers from microbial fermentation is a less expensive, adaptable, and environmentally harmless process when compared to producing plastics made from fossil fuels. However, bioplastics are susceptible to water hydrolysis, which reduces their strength and durability [161,162][29][30]. The synthesis of bioplastics, such as polylactic acid (PLA) and polyhydroxyalkanoates (PHAs), derives from renewable sources such as food waste, lignocellulose residues, and vegetable oils [161,162][29][30]. The purpose of studies concerning bioplastic production through microbial fermentation is to create plastic with mechanical, physical-chemical, and thermal properties to match the performance of fossil-based plastics. On the other hand, the high biodegradability of bioplastic makes it environmentally friendly, reducing plastic waste due to its shorter product lifetime [162,163][30][31].
Although it is possible to produce synthetic biodegradable polymers from petrochemical byproducts such as polycaprolactone (PCL) [164[32][33],165], several methods of bioplastic synthesis from renewable resources use natural polysaccharides (lignin, cellulose, and starch), proteins, and lipids. Other examples include polyhydroxyalkanoates (PHA) [154[22][34],166], polyhydroxy butyrate (PHB) [155][23], and poly(3-hydroxybutyrate-co 3-hydroxy valerate) (PHBV) [158,167][26][35]. The third alternative uses microbiological fermentation, whereby lactic acid is synthesized and polymerized to form a PLA-biobased product [14][36].
Under biorefinery concepts, wine lees (the liquid and solid residual fractions, remaining streams, and alcohol-free nutrient-rich liquid) from producing Merlot red wine were employed as a bioresource to produce bioplastic poly(3-hydroxybutyrate) (PHB) synthesis. This included the production of generic fermentation feedstock (ethanol, phenolic compounds, and tartaric acid) [97][7]. Thus, the crude nutrient-rich hydrolysate from enzymatic hydrolysis was used to initiate PHB production with high efficiency using the strain Cupriavidus necator DSM 7237, while the hydrolysis of pretreated lees was performed using crude enzyme consortia via solid-state fermentation with Aspergillus oryzae [97][7].
Demirci et al. (2019) and Jung et al. (2022) produced paramylon and xanthan gum biopolymers from fermentable sugars obtained and extracted from bread waste (stale bread that remained unsold and returned goods from shops). After the enzymatic hydrolysis step, the glucose thus obtained was employed in both studies as a bioresource: i) as a carbon substrate for the heterotrophic cultivation of the microalgae Euyglena gracilis to produce the carbohydrate paramylon [80][37]; ii) as a carbon source in the fermentation of Xanthomonas spp. to obtain the xanthan gum polysaccharide [82][38]. Xanthan gum is widely used in the food industry as a common additive to stabilize and emulsify food products.
Although up to 50% of residual whey from cheesemaking is being valorized as a source of high-added-value compounds in food/pharmaceutical factories, mainly as proteins with high levels of biological properties, lactose, lactic acid (LA), and minerals, there is still a high proportion of this whey that is discarded, thus promoting environmental pollution [168][39]. An integrated cheese whey valorization process [69][11] utilized the wastewater from cheesemaking to produce several added-value products, including lipid recovery via thermocalcic precipitation, protein recovery via ultrafiltration, and lactose valorization through biological processes to obtain polyhydroxyalkanoates (PHA). Therefore, this circular economic approach can provide lactose as a carbon source for biomolecules, lipids, and proteins to produce bioplastic PHA for use as a flame retardant, along with whey protein concentrates for the food industry. It can also potentially obtain lactic acid (LA), a monomer of PLA derived from natural sources. Thus, LA is produced using the bacterial fermentation of fermentable biomass. Likewise, reducing sugars from bread wastes drawn from Indian bakery wastes were simultaneously submitted to saccharification and SSF by LAB, suggesting bread wastes as potential candidates for lactic acid production [84][40]. Those findings are interesting because PLA (C3H4O2)n is a biodegradable thermoplastic that can be synthesized using residues such as sugarcane bagasse, corn cobs, starch, and food waste [42,169][41][42]. PLA is a well-known biopolymer with many applications and properties, such as biodegradability, biocompatibility, elasticity, moldability, and rigidity. In addition, it is the first polymer produced from renewable sources to be marketed for various applications, such as for grocery bags and food packaging [42][41]. PLA synthesis has, as an initial step, the production of LA by the fermentation process. Lactic acid comes in the form of a white powder or yellow liquid. Its structural formula incorporates chiral carbon, which leads to the formation of a left-handed (L+) and a right-handed (D−) structure. The chemical activity of the LA molecule depends on the acidity in an aqueous medium and the functional activities of groups present in the chemical structure, such as the carboxyl and hydroxyl groups, which promote a wide variety of chemical reactions [13,169][42][43]. The global market for PLA is constantly expanding, which causes an increase in its demand due to its widespread use in the packaging, agriculture, and transportation industries. Therefore, an efficient manufacturing method with reduced production costs is needed so as to evaluate other methods that can be employed when using agricultural and food waste [161][29].
4. Nanomaterials
Nanotechnology is being applied in several areas and sectors of industry, such as food, cosmetics, and chemistry. Some studies have demonstrated the importance of nanomaterials based on fermented food residues or byproducts and several applications on health and food. A nanocomposite based on kefiran films has garnered research attention due to the electrospinning and film-forming abilities of the kefiran polysaccharides recovered from kefir grains. Kefiran, an exopolysaccharide, derived from the microflora of kefir grains, which are used to produce fermented milk beverages, has recently attracted research attention due to its biological properties and its potential for use in the nanomedicine and food packaging industries [30][44]. Recent investigations reported a kefiran biopolymer, manufactured via electrospinning, to produce kefiran nanofiber [170,171][45][46]. Kefiran nanofibersis potentially valuable for encapsulating bioactive ingredients in food [172][47], as matrix for probiotic/drug delivery [72[48][49][50][51][52],73,74,173,174], andscaffolds for regenerative medicine and tissue engineering [170][45].
5. Other Applications Using Fermentative Residues
Fermentation residues from Ca/Fe-rich antibiotics, for example, can be considered very hazardous but, at the same time, can be recycled and applied in adsorption resources by using biochar [175][53]. Despite the possibilities, such hazardous wastes have seldom been explored and require several stages of waste preparation. In one study, residual vancomycin and antibiotic-resistant genes were fully exposed during pyrolysis. The process showed fast kinetics and a maximum adsorption rate of 102 mg p/g. Ca and Fe represented active sites that helped in the adsorption process. Therefore, the use of biochar produced from fermentative residues can be used in the treatment of wastewater to absorb contaminants. Furthermore, biochar can be used as a phosphate fertilizer, as it promotes seed germination (germination rate: 96.7% vs. 80.0% in the control group, p < 0.01) and seedling growth (shoot length was increased by 57.9%, p < 0.01) due to the slow release of the available phosphate. Consequently, hazardous waste was turned into phosphate fertilizer, adding to the benefits of biomass reutilization, and recovering phosphate from wastewater. Biochar from spiramycin (SPI) fermentation residues (SFR) in China, which are dangerous, can also be cited here. The pyrolysis method was adopted to convert SFR to biochar to remove SPI from wastewater, and the results showed no residual SPI, indicating the achievement of SFR detoxification. Furthermore, after recycling 5 times, the SPI removal efficiency was still greater than 80.0%, indicating a promising method for SFR disposal [176][54].
Lipstatin, a fermentative residue (LFR) from the pharmaceutical industry, can be discarded in the soil after the composting stage due to its high content of organic matter. Generally, residues from such fermentation processes are composed of a high amount of organic matter, which is of high importance in fertilization. The pH value of soil fertilized with composted LFR slightly decreased, without the accumulation of lipstatin. Soil nutrients, including the available phosphorus, potassium, organic matter, and soluble organic matter, increased significantly in soil fertilized with composted LFR, providing excellent application as a biofertilizer [177][55]. Likewise, the synthesis of doramectin offers another interesting example of the use of fermentative residue from the pharmaceutical industry, which generates a high rate of residues with a large amount of organic matter, resulting in their applicability in agricultural fertilization [178][56].
References
- Suescun–Ospina, S.T.; Ávila–Stagno, J.; Vera-Aguilera, N.; Astudillo-Neira, R.; Trujillo-Mayol, I.; Alarcón-Enos, J. Effects of Drying Method on Bioactive Compounds Contents, Rumen Fermentation Parameters and in Vitro Methane Output of Waste Dried País Grape (Vitis vinifera L.) Marc. Food Biosci. 2023, 51, 102154.
- Samray, M.N.; Masatcioglu, T.M.; Koksel, H. Bread Crumbs Extrudates: A New Approach for Reducing Bread Waste. J. Cereal. Sci. 2019, 85, 130–136.
- Pfleger, B.F.; Takors, R. Recent Progress in the Synthesis of Advanced Biofuel and Bioproducts. Curr. Opin. Biotechnol. 2023, 80, 102913.
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J. Microbiology of Synthesis Gas Fermentation for Biofuel Production. Curr. Opin. Biotechnol. 2007, 18, 200–206.
- Narisetty, V.; Nagarajan, S.; Gadkari, S.; Ranade, V.V.; Zhang, J.; Patchigolla, K.; Bhatnagar, A.; Kumar Awasthi, M.; Pandey, A.; Kumar, V. Process Optimization for Recycling of Bread Waste into Bioethanol and Biomethane: A Circular Economy Approach. Energy Convers. Manag. 2022, 266, 115784.
- Narisetty, V.; Zhang, L.; Zhang, J.; Sze Ki Lin, C.; Wah Tong, Y.; Loke Show, P.; Kant Bhatia, S.; Misra, A.; Kumar, V. Fermentative Production of 2,3-Butanediol Using Bread Waste—A Green Approach for Sustainable Management of Food Waste. Bioresour. Technol. 2022, 358, 127381.
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.; Koutinas, A.A. Wine Lees Valorization: Biorefinery Development Including Production of a Generic Fermentation Feedstock Employed for Poly(3-Hydroxybutyrate) Synthesis. Food Res. Int. 2015, 73, 81–87.
- Jugwanth, Y.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorization of Sugarcane Bagasse for Bioethanol Production through Simultaneous Saccharification and Fermentation: Optimization and Kinetic Studies. Fuel 2020, 262, 116552.
- Hazeena, S.H.; Shurpali, N.J.; Siljanen, H.; Lappalainen, R.; Anoop, P.; Adarsh, V.P.; Sindhu, R.; Pandey, A.; Binod, P. Bioprocess development of 2, 3-butanediol production using agro-industrial residues. Bioprocess. Biosyst. Eng. 2022, 45, 1527–1537.
- Nagai, H.; Kobayashi, M.; Tsuji, Y.; Nakashimada, Y.; Kakizono, T.; Nishio, N. Biological and Chemical Treatment of Solid Waste from Soy Sauce Manufacture. Water Sci. Technol. 2002, 45, 335–338.
- Bosco, F.; Carletto, R.A.; Marmo, L. An Integrated Cheese Whey Valorization Process. Chem. Eng. Trans. 2018, 64, 379–384.
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas Fermentation Process Development for Production of Biofuels and Chemicals: A Review. Bioresour. Technol. Rep. 2019, 7, 100279.
- Adessi, A.; Venturi, M.; Candeliere, F.; Galli, V.; Granchi, L.; De Philippis, R. Bread Wastes to Energy: Sequential Lactic and Photo-Fermentation for Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 9569–9576.
- Yu, X.; Chen, G.; Widenmeyer, M.; Kinski, I.; Liu, X.; Kunz, U.; Schüpfer, D.; Molina-Luna, L.; Tu, X.; Homm, G.; et al. Catalytic Recycling of Medical Plastic Wastes over La0.6Ca0.4Co1–Fe O3− Pre-Catalysts for Co-Production of H2 and High-Value Added Carbon Nanomaterials. Appl. Catal. B 2023, 334, 122838.
- Anniwaer, A.; Chaihad, N.; Choirun Az Zahra, A.; Kurnia, I.; Kasai, Y.; Kongparakul, S.; Samart, C.; Kusakabe, K.; Abudula, A.; Guan, G. Utilization of Fruit Waste for H2-Rich Syngas Production via Steam Co-Gasification with Brown Coal. Carbon Resour. Convers. 2023, 6, 315–325.
- Mateus, S.; Carvalheira, M.; Cassidy, J.; Freitas, E.; Oehmen, A.; Reis, M.A.M. Two-Stage Anaerobic Digestion System Treating Different Seasonal Fruit Pulp Wastes: Impact on Biogas and Hydrogen Production and Total Energy Recovery Potential. Biomass Bioenergy 2020, 141, 105694.
- Ishak, M.A.M.; Ani, A.Y.; Syed Ismail, S.N.A.; Ali, M.L.M.; Ahmad, R. Conversion of Biomass to Biofuels. In Value-Chain of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–67.
- Villanueva-Galindo, E.; Vital-Jácome, M.; Moreno-Andrade, I. Dark Fermentation for H2 Production from Food Waste and Novel Strategies for Its Enhancement. Int. J. Hydrogen Energy 2023, 48, 9957–9970.
- Lu, C.; Wang, G.; Zhang, Q.; Yang, X.; Yu, J.; Liu, T.; Petracchini, F.; Zhang, Z.; Sun, Y.; Jiang, D.; et al. Comparison of Biorefinery Characteristics: Photo-Fermentation Biohydrogen, Dark Fermentation Biohydrogen, Biomethane, and Bioethanol Production. Appl. Energy 2023, 347, 121463.
- Ahmad, A.; K, R.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen Production through Dark Fermentation: Recent Trends and Advances in Transition to a Circular Bioeconomy. Int. J. Hydrogen Energy 2023, in press.
- Maina, S.; Schneider, R.; Alexandri, M.; Papapostolou, H.; Nychas, G.-J.; Koutinas, A.; Venus, J. Volumetric Oxygen Transfer Coefficient as Fermentation Control Parameter to Manipulate the Production of Either Acetoin or D-2,3-Butanediol Using Bakery Waste. Bioresour. Technol. 2021, 335, 125155.
- Walker, T.R.; Fequet, L. Current Trends of Unsustainable Plastic Production and Micro(Nano)Plastic Pollution. TrAC Trends Anal. Chem. 2023, 160, 116984.
- Plastics Europe. Plastic-the Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 21 September 2023).
- Statista Research Department. Statista Annual Production of Plastics Worldwide from 1950 to 2021. 2022. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 21 September 2023).
- GRID Arendal. Global Plastic Production and Accumulation. 2021. Available online: https://www.grida.no/resources/1504.1 (accessed on 21 September 2023).
- Hathi, Z.J.; Haque, M.A.; Priya, A.; Qin, Z.; Huang, S.; Lam, C.H.; Ladakis, D.; Pateraki, C.; Mettu, S.; Koutinas, A.; et al. Fermentative Bioconversion of Food Waste into Biopolymer Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Using Cupriavidus Necator. Environ. Res. 2022, 215, 114323.
- Hees, T.; Zhong, F.; Stürzel, M.; Mülhaupt, R. Tailoring Hydrocarbon Polymers and All-Hydrocarbon Composites for Circular Economy. Macromol. Rapid Commun. 2019, 40, 1800608.
- Zhong, S.; Pearce, J.M. Tightening the Loop on the Circular Economy: Coupled Distributed Recycling and Manufacturing with Recyclebot and RepRap 3-D Printing. Resour. Conserv. Recycl. 2018, 128, 48–58.
- Sohn, Y.J.; Kim, H.T.; Baritugo, K.; Jo, S.Y.; Song, H.M.; Park, S.Y.; Park, S.K.; Pyo, J.; Cha, H.G.; Kim, H.; et al. Recent Advances in Sustainable Plastic Upcycling and Biopolymers. Biotechnol. J. 2020, 15, 1900489.
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501.
- Bhatia, S.K.; Otari, S.V.; Jeon, J.-M.; Gurav, R.; Choi, Y.-K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Rajesh Banu, J.; Yoon, J.-J.; et al. Biowaste-to-Bioplastic (Polyhydroxyalkanoates): Conversion Technologies, Strategies, Challenges, and Perspective. Bioresour. Technol. 2021, 326, 124733.
- Oh, Y.-R.; Jang, Y.-A.; Song, J.K.; Eom, G.T. Efficient Enzymatic Depolymerization of Polycaprolactone into 6-Hydroxyhexanoic Acid by Optimizing Reaction Conditions and Microbial Conversion of 6-Hydroxyhexanoic Acid into Adipic Acid for Eco-Friendly Upcycling of Polycaprolactone. Biochem. Eng. J. 2022, 185, 108504.
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Anaerobic Biodegradation Tests of Poly(Lactic Acid) and Polycaprolactone Using New Evaluation System for Methane Fermentation in Anaerobic Sludge. Polym. Degrad. Stab. 2009, 94, 1397–1404.
- Lanfranchi, A.; Tassinato, G.; Valentino, F.; Martinez, G.A.; Jones, E.; Gioia, C.; Bertin, L.; Cavinato, C. Hydrodynamic Cavitation Pre-Treatment of Urban Waste: Integration with Acidogenic Fermentation, PHAs Synthesis and Anaerobic Digestion Processes. Chemosphere 2022, 301, 134624.
- Kerketta, A.; Vasanth, D. Madhuca Indica Flower Extract as Cheaper Carbon Source for Production of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Using Ralstonia Eutropha. Process Biochem. 2019, 87, 1–9.
- Xiao, X.; Li, S.; Zhou, X.; Li, M.; Zhang, Y.; Ye, H. The Anti-Obesogenic Effects and Underpinning Mechanisms of Fermented Plant-Based Foods: A Review. Trends Food Sci. Technol. 2023, 136, 1–10.
- Jung, J.-M.; Kim, J.Y.; Kim, J.-H.; Kim, S.M.; Jung, S.; Song, H.; Kwon, E.E.; Choi, Y.-E. Zero-Waste Strategy by Means of Valorization of Bread Waste. J. Clean Prod. 2022, 365, 132795.
- Demirci, A.S.; Palabiyik, I.; Apaydın, D.; Mirik, M.; Gumus, T. Xanthan Gum Biosynthesis Using Xanthomonas Isolates from Waste Bread: Process Optimization and Fermentation Kinetics. LWT 2019, 101, 40–47.
- Barba, F.J. An Integrated Approach for the Valorization of Cheese Whey. Foods 2021, 10, 564.
- Sadaf, A.; Kumar, S.; Nain, L.; Khare, S.K. Bread Waste to Lactic Acid: Applicability of Simultaneous Saccharification and Solid State Fermentation. Biocatal. Agric. Biotechnol. 2021, 32, 101934.
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Review on Biodegradable Polylactic Acid (PLA) Production from Fermentative Food Waste—Its Applications and Degradation. Int. J. Biol. Macromol. 2023, 234, 123703.
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)—Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715.
- Gautam, K.; Vishvakarma, R.; Sharma, P.; Singh, A.; Kumar Gaur, V.; Varjani, S.; Kumar Srivastava, J. Production of Biopolymers from Food Waste: Constrains and Perspectives. Bioresour. Technol. 2022, 361, 127650.
- de Carvalho, A.P.A.; Conte-Junior, C.A. Food-Derived Biopolymer Kefiran Composites, Nanocomposites and Nanofibers: Emerging Alternatives to Food Packaging and Potentials in Nanomedicine. Trends Food Sci. Technol. 2021, 116, 370–386.
- Mehrali, F.; Ziyadi, H.; Hekmati, M.; Faridi-Majidi, R.; Qomi, M. Kefiran/Poly(Vinyl Alcohol)/Poly(Vinyl Pyrrolidone) Composite Nanofibers: Fabrication, Characterization and Consideration of Effective Parameters in Electrospinning. SN Appl. Sci. 2020, 2, 895.
- Esnaashari, S.S.; Rezaei, S.; Mirzaei, E.; Afshari, H.; Rezayat, S.M.; Faridi-Majidi, R. Preparation and Characterization of Kefiran Electrospun Nanofibers. Int. J. Biol. Macromol. 2014, 70, 50–56.
- Jenab, A.; Roghanian, R.; Emtiazi, G.; Ghaedi, K. Manufacturing and Structural Analysis of Antimicrobial Kefiran/Polyethylene Oxide Nanofibers for Food Packaging. Iran. Polym. J. 2017, 26, 31–39.
- Jenab, A.; Roghanian, R.; Emtiazi, G. Encapsulation of Platelet in Kefiran Polymer and Detection of Bioavailability of Immobilized Platelet in Probiotic Kefiran as a New Drug for Surface Bleeding. J. Med. Bacteriol. 2015, 4, 45–55.
- Piermaria, J.; Diosma, G.; Aquino, C.; Garrote, G.; Abraham, A. Edible Kefiran Films as Vehicle for Probiotic Microorganisms. Innov. Food Sci. Emerg. Technol. 2015, 32, 193–199.
- Gagliarini, N.; Diosma, G.; Garrote, G.L.; Abraham, A.G.; Piermaria, J. Whey Protein-Kefiran Films as Driver of Probiotics to the Gut. LWT 2019, 105, 321–328.
- Dadashi, S.; Boddohi, S.; Soleimani, N. Preparation, Characterization, and Antibacterial Effect of Doxycycline Loaded Kefiran Nanofibers. J. Drug Deliv. Sci. Technol. 2019, 52, 979–985.
- Blandón, L.M.; Islan, G.A.; Castro, G.R.; Noseda, M.D.; Thomaz-Soccol, V.; Soccol, C.R. Kefiran-Alginate Gel Microspheres for Oral Delivery of Ciprofloxacin. Colloids Surf. B Biointerfaces 2016, 145, 706–715.
- Zhang, M.; Chen, Q.; Zhang, R.; Zhang, Y.; Wang, F.; He, M.; Guo, X.; Yang, J.; Zhang, X.; Mu, J. Pyrolysis of Ca/Fe-Rich Antibiotic Fermentation Residues into Biochars for Efficient Phosphate Removal/Recovery from Wastewater: Turning Hazardous Waste to Phosphorous Fertilizer. Sci. Total Environ. 2023, 869, 161732.
- Gao, T.; Shi, W.; Zhao, M.; Huang, Z.; Liu, X.; Ruan, W. Preparation of Spiramycin Fermentation Residue Derived Biochar for Effective Adsorption of Spiramycin from Wastewater. Chemosphere 2022, 296, 133902.
- Xiao, J.; Wang, G.; Liu, H.; Dai, X. Application of Composted Lipstatin Fermentation Residue as Organic Fertilizer: Temporal Changes in Soil Characteristics and Bacterial Community. Chemosphere 2022, 306, 135637.
- Pan, M.; Xin, Y.; Wang, Z.; Jia, W.; Lu, H.; Jiang, S.; Wu, Z.; Chen, X.; Wang, Q.; Du, H.; et al. Benign Treatment and Resource Utilization Characteristics of Doramectin Fermentation Residues. J. Clean. Prod. 2023, 401, 136777.
More
