Many algae respond to the CO2 limitation in seawater by inducing a CO2 concentrating mechanism (CCM) to obtain sufficient inorganic carbon to meet their photosynthetic needs. To assess the diversity of the CCM functions and activities in different algae, reliable metrics to measure and quantify the relative functions of CCM need to be established. As reported by Badger et al. [47], we screened out several indicators that may be suitable for measuring the CCM of macroalgae were demonstrated.
- CO2 concentrating mechanism
- Ulva sp.
- green tide
- inorganic carbon transporters
- carbonic anhydrase
- Rubisco
1 CO2 affinity of photosynthesis versus Rubisco1. CO Affinity of Photosynthesis Versus Rubisco
Rubisco’s low affinity for external CO2 drove the evolution of CCMs. Therefore, one of the most useful indicators of whether a photosynthetic organism has a CCM is to compare the affinity of photosynthesis for external CO2 with the affinity of Rubisco for CO2. This approach was originally applied to higher plants, and by measuring the affinity Km (Rubisco CO2) of Rubisco extracted from photosynthetic organisms to substrate CO2, it was inferred that C3 species might require a CCM [69][1]. However, due to the limitation of extraction technology, the affinity determination of photosynthetic organism Rubisco lacked accuracy. With the improvement of extraction and analysis techniques, this method has become widely used to evaluate algal CCMs. In general, the researchers measured the photosynthetic oxygen evolution rate under different inorganic carbon concentrations, and used the data analysis software to fit the inorganic carbon response curve (P-C curve) by using Michaelis-Menten equation, where Km is the substrate concentration (DIC concentration) when the photosynthetic rate is half of the maximum, from which we can infer the affinity of algae photosynthesis to CO2 Km (photosynthetic CO2). Since marine algae may experience more persistent CO2 limitations than fresh- and brackish-water autotrophs, it can be assumed that the type of water environment determines the effectiveness of CCM. Comparing the in vivo photosynthesis and the in vitro carboxylation of Rubisco under ambient oxygen levels can show its ability to concentrate CO2 exceeds the level of environmental CO2 [70][2]. For example, C. reinhardtii’s ratio of Km (photosynthetic CO2) to Km (Rubisco CO2) is approximately 1:30, and that of Ulva sp. is about 5–10:68 [1[3][4][5],71,72], both apparently rely on CCM to improve the efficiency of Rubisco, whereas other algae with close specific gravity may not use CCM.
2 Effects of CA inhibitors2. Effects of CA Inhibitors
Because CA is central in all chloroplast CCM models, determining the effect of CA inhibitors is useful in studying the functional aspects of algal CCM. This is especially true for chloroplast-penetrating inhibitors like EZA (Ethoxzolamide), which are likely to inhibit most forms of internal CA. EZA is often used to obtain low inorganic carbon affinity for photosynthesis of algae. If the algae showed low inorganic carbon affinity under the action of EZA, it is proved that there is intracellular CA-mediated CCM in the algae, but no effect may not be the conclusive evidence of CCM deletion. For CA inhibitors that cannot penetrate cell membranes, such as AZA (Acetazolamide), only apply to inhibit the activity of external CA [47][6], the effect on the function of chloroplast CCM is less easily explained. By comparing the relative effects of EZA and AZA, the relative contributions of internal and external CA forms to photosynthetic Ci absorption can be determined (Fig. 3Figure 1). For example, in the study of Gao et al. [73][7], after adding AZA the net photosynthetic rate of U. linza decreased, and the inhibition rate was noted to be 26.26%. Compared with AZA, EZA demonstrated a higher inhibition rate of photosynthesis (75.19%), which indicated that the internal CA contributed more to the absorption of photosynthesis. Xu et al. [74][8] found that U. linza and U. prolifera have obvious extracellular and intracellular CA activity by using different CA inhibitors. However, extracellular CA enzyme activity itself accelerates the mutual conversion between HCO3− and CO2, but cannot affect the CO2 equilibrium concentration and CO2 mainly enters the cell through passive diffusion. This indicates that HCO3−-utilization in this manner does not function well at high pH (pH > 9.4) because the equilibrium concentration of CO2 is low. It is obvious that in addition to the extracellular CA catalytic utilization of HCO3−, there must be another means of using HCO3− in U. prolifera.
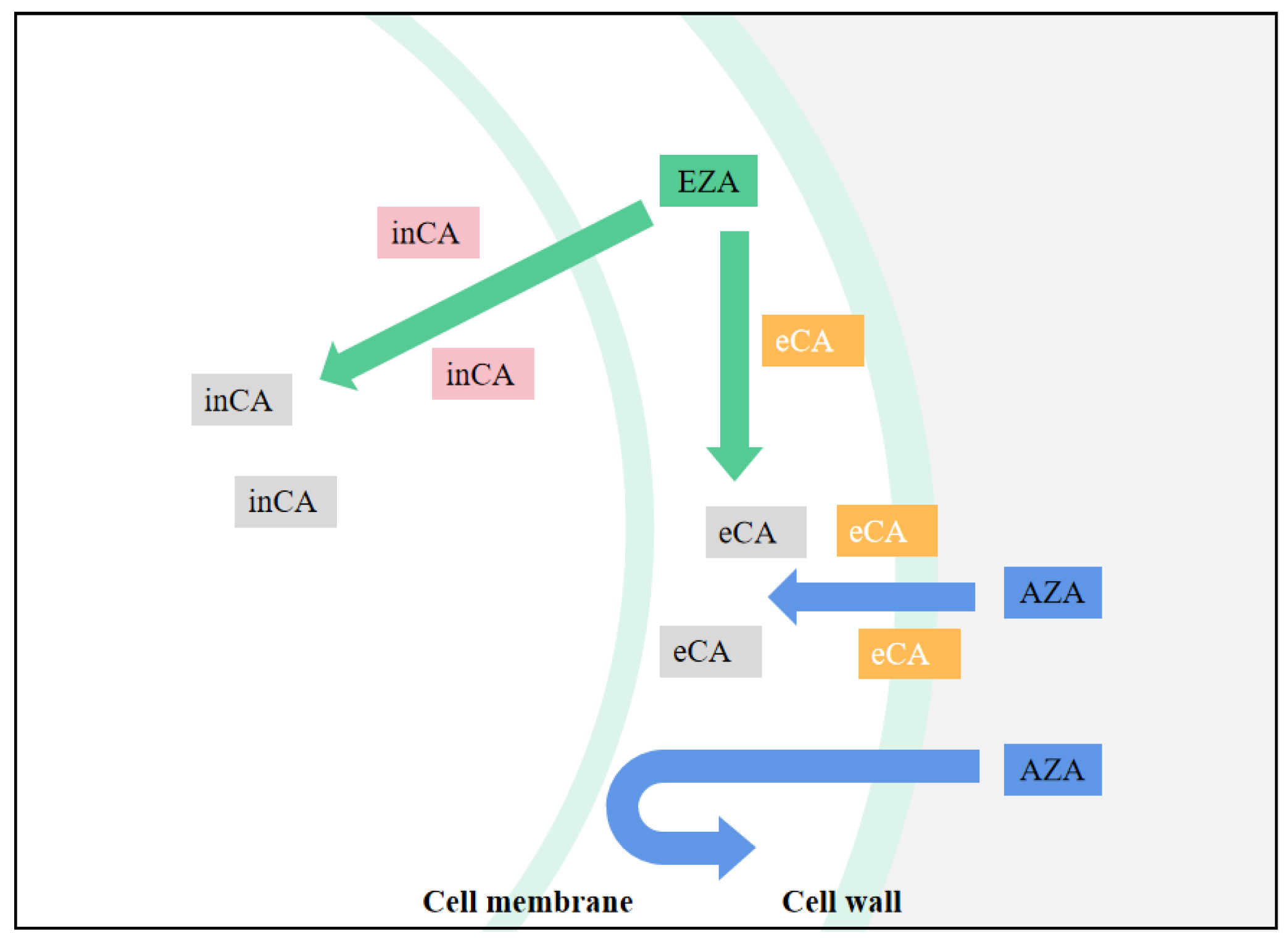
Figure 31.
Schematic diagram of CA inhibitor actions. inCA means intracellular CA, eCA means extracellular CA, and when the CA icon is gray, CA is suppressed.
3 Using HCO3− as photosynthetic substrate3. Using HCO as Photosynthetic Substrate
The function of HCO3− in photosynthesis has been perceived as a property of algae [75][9]. The utilization of HCO3− by algae depends on the involvement of external CA and plasma membrane transporters [76-78][10][11][12]. The use of HCO3− is strongly correlated with the presence of a CCM. However, some macroalgae are not only capable of utilizing HCO3− as a carbon source, some have demonstrated the use of CO2 in specific situations. Through the detection of δ13C isotopes in Gracilaria and Ulva, it was found that when the concentration of environmental CO2 was high, there was a physiological transition from using HCO3− almost exclusively to predominantly using CO2. At the current seawater pCO2, many macroalgae use HCO3− rather than dissolved CO2, and utilize CA to convert HCO3− to CO2 for use by Rubisco [79-82][13][14][15][16]. For example, Mercado et al. [83][17] found that under the current seawater CO2 level, the chlorophyll plants U. rigida and U. compressa cannot obtain sufficient CO2 through diffusion absorption alone; therefore, a CCM must be used to obtain HCO3−. However, macroalgae may downregulate their CCM, reduce HCO3− use, and become dependent on CO2 as the main carbon source when CO2 concentrations are high [1,84-86][3][18][19][20]. Consequently, when using these indicators to evaluate the CCM of Ulva sp., there may be a decrease in the use of HCO3− in certain circumstances, where there is still CCM activity but with a certain degree of downregulation.
4 Changes in affinity to external Ci depends on growth Ci conditions4. Changes in Affinity to External Ci Depends on Growth Ci Conditions
When there is Ci limitation in the external environment, the affinity of the Ci transporter for external CO2 and HCO3- increases, and the intracellular and extracellular CA activity also increases. The two work together to increase the affinity of microalgae for external Ci by more than 10 times [76][10]. This inducible change appears to be strong evidence that cellular infrastructure is involved in supplying CO2 to Rubisco in chloroplasts These inducible CCM are not limited to microalgae but have been observed in many macroalgae as well, including Ulva [1][3], Gracilaria [87][21], Porphyra [88][22] and Fucus [89][23]. That is, some algae may have an inducible CCM to meet their environmental needs when facing external Ci periodic constraints, while other algae may not have such a flexible arrangement.
References
- Lilley, R.M.; Walker, D.A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975, 55, 1087–1092.
- Capó-Bauçà, S.; Iñiguez, C.; Aguiló-Nicolau, P.; Galmés, J. Correlative adaptation between Rubisco and CO2-concentrating mechanisms in seagrasses. Nat. Plants 2022, 8, 706–716.
- Björk, M.; Haglund, K.; Ramazanov, Z.; Pedersén, M. Inducible mechanisms for HCO3− utilization and repression of photorespiration in protoplasts and thalli of three species of Ulva (Chlorophyta). J. Phycol. 1993, 29, 166–173.
- Beer, S.; Israel, A.; Drechsler, Z.; Cohen, Y. Photosynthesis in Ulva fasciata. V. Evidence for an inorganic carbon concentrating system, and ribulose-1,5-bisphosphate carboxylase/oxygenase CO2 kinetics. Plant Physiol. 1990, 94, 1542–1546.
- Axelsson, L.; Ryberg, H.; Beer, S. Two modes of bicarbonate utilization in the marine green macroalga Ulva lactuca. Plant Cell Environ. 1995, 18, 439–445.
- Badger, M.; Andrews, T.J.; Whitney, S.M.; Ludwig, M.; Price, G.D. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplastbased CO2-concentrating mechanisms in algae. Can. J. Bot. 1998, 76, 1052–1071.
- Gao, G.; Liu, Y.; Li, X.; Feng, Z.; Xu, J. An ocean acidification acclimatised green tide alga is robust to changes of seawater carbon chemistry but vulnerable to light stress. PLoS ONE 2016, 11, e0169040.
- Xu, J.T.; Wang, X.; Zhong, Z.; Yao, D. The mechanism of the characters of inorganic carbon acquisition to temperature in two Ulva species. Sheng Tai Xue Bao 2013, 33, 7892–7897. (In Chinese)
- Lucas, W.J. Photosynthetic assimilation of exogenous HCO3− by aquatic plants. Annu. Rev. Plant Physiol. 1983, 34, 71–104.
- Badger, M.R. The CO2-concentrating mechanism in aquatic phototrophs. Photosynthesis 1987, 10, 219–274.
- Johnston, A.M. The acquisition of inorganic carbon by marine macroalgae. Can. J. Bot. 1991, 69, 1123–1132.
- Badger, M.R.; Price, G.D. The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiol. Plant 1992, 84, 606–615.
- Gao, K.; McKinley, K.R. Use of macroalgae for marine biomass production and CO2 remediation: A review. J. Appl. Phycol. 1994, 6, 45–60.
- Israel, A.; Hophy, M. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Global Change Biol. 2002, 8, 831–840.
- Badger, M.R. The role of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth. Res. 2003, 77, 83–94.
- Koch, M.S.; Bowes, G.; Ross, C.; Zhang, X. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biol. 2013, 19, 103–132.
- Mercado, J.M.; Gordillo, F.J.; Figueroa, F.L.; Niell, F.X. External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. J. Exp. Mar. Biol. Ecol. 1998, 221, 209–220.
- Gao, K.; Aruga, Y.; Asada, K.; Kiyohara, M. Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. And G. chilensis. J. Appl. Phycol. 1993, 5, 563–571.
- Xu, Z.; Zou, D.; Gao, K. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Bot. Mar. 2010, 53, 123–129.
- Cornwall, C.E.; Hepburn, C.D.; Pritchard, D.; Currie, K.I.; McGraw, C.M.; Hunter, K.A.; Hurd, C.L. Carbon-Use Strategies in Macroalgae: Differential Responses to Lowered pH and Implications for Ocean Acidification. J. Phycol. 2012, 48, 137–144.
- García-Sánchez, M.J.; Fernández, J.A.; Niell, X. Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 1994, 194, 55–61.
- Mercado, J.M.; Niell, F.X.; Figueroa, F.L. Regulation of the mechanism for HCO3− use by the inorganic carbon level in Porphyra leucosticta Thur in le Jolis (Rhodophyta). Planta 1997, 201, 319–325.
- Johnston, A.M.; Raven, J.A. Effects of culture in high CO2 on the photosynthetic physiology of Fucus serratus. Br. Phycol. J. 1990, 25, 75–82.
