1. Introduction
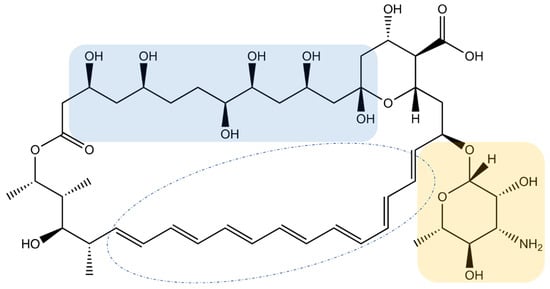
Amphotericin B (AmB) is the leading compound of the polyene macrolide family, so named because of the numerous conjugated double bonds in a large macrolactone ring (Figure 1). Its structure also contains a polyol domain and a deoxysugar mycosamine group.
Figure 1.
Molecular structure of AmB. Blue zone: polyol domain, yellow zone: deoxysugar mycosamine group.
AmB is an old molecule as it was first discovered and extracted in the 1950s in Venezuela from
Streptomyces nodosus [1][2][1,2]. The molecule rapidly reached the market after the FDA approved it in 1958
[2]. AmB is considered to have a broad spectrum of activity not only on fungi (i.e., filamentous, molds, yeasts, etc.) but also on parasites (e.g., Leishmania). Thus, AmB is efficient against different fungal genera/species:
Candida spp.,
Aspergillus spp.,
Histoplasma capsulatum,
Coccidioides immitis,
Blastomyces dermatitidis,
Rhodotorula spp.,
Cryptococcus neoformans,
Sporothrix schenkii,
Fusarium spp.,
Cladosporium spp.,
Scytalidium spp., and Zygomycetes. Conversely, the genera/species
Candida lusitaniae,
Candida auris,
Trichosporon spp.,
Geotrichum spp.,
Scedosporium spp.,
Fusarium spp., and
Aspergillus terreus are resistant or less sensitive to this molecule
[3][4][3,4]. It should be noted that resistance to polyenes still remains rare (i.e., compared to resistance to other antifungal drugs, such as azoles). Furthermore, although several mechanisms of resistance to polyenes have been described in the literature, the main mechanism of resistance remains associated with a modification in the sterol composition at the level of the cell membrane or even a depletion of ergosterol, attributable to gene-level mutations involved in its biosynthesis
[5][6][5,6]. Noteworthily, it is more and more common to find conflicting data regarding the activity of AmB against different fungal species/strains in the literature
[4].
The affinity of AmB for the ergosterol of the membranes of microorganisms gives it its selective microbial activity. This selectivity is only slightly higher compared to that of cholesterol from mammalian cell membranes, making its therapeutic efficacy very narrow
[4][5][6][4,5,6]. Considering the structure of the compound, studies demonstrated that the dimers forming AmB are toxic for eukaryotic cells. While the polyaggregated forms present reduced resistance for host cells, they retain antiparasitic activity at the same time
[7]. AmB is mainly used in monotherapy, rarely as first-line, except for in the management of serious systemic fungal infections. AmB can also be used in combination with other antifungals such as flucytosine or fluconazole depending on particular clinical situations
[8]. However, treatment with AmB is not devoid of side effects, which occur in 25 to 90% of patients
[3][9][3,9]. The reported symptoms range from infusion-related reactions up to anaphylaxis, which can be prevented by drugs (e.g., corticoids, antihistamines, analgesics, etc.). Another serious side effect is a significant risk of nephrotoxicity which limits its use
[10]. The formulation of AmB is an important topic of research with the aim to develop forms which improve its therapeutic effect and lead to less nephrotoxicity
[11][12][11,12]. All the formulations are based on lipidic compounds mixed with AmB due to the amphiphilic nature of the antifungal. The lipid formulations of AmB which have been developed are either AmB in a colloidal dispersion, or AmB in a lipid complex or liposomal AmB. Thus, these three formulations differ in their lipid composition and therefore in their physical and pharmacokinetic characteristics, their efficacy, and their tolerance to efficacy
[13]. Evidence has been shown that self-assembled mixed micelles containing AmB based on a combination of lecithin with polymers have reduced in vitro cytotoxicity and improved AmB solubility results with increased parenteral and oral bioavailability in rats compared to Fungizone
® [14]. Moreover, the oral administration of AmB encapsulated in nanoparticles (N-palmitoyl-N-methyl-N,N-dimethyl-N,N,N-trimethyl-6-O-glycol chitosan) has also showed high efficacy in mouse models of candidiasis, aspergillosis or visceral leishmaniasis compared to AmBisome
® administered parenterally
[15]. Data have highlighted that using the lipid-based formulation of AmB is more expensive than conventional micellar deoxycholate AmB, which is why its use is limited in clinical practice
[3][4][3,4]. This evidence has been discussed in numerous bibliographic reviews presenting the various formulations of AmB. The development of an orally active formulation of AmB capable of reducing the systemic drug toxicity, avoiding infusion-related adverse events, improving patient compliance, and reducing the costs associated with the intravenous administration of commercial formulations of AmB is an urgent requirement. Up until now, in contrast to lipid formulations, no inorganic nanoparticles, as an agent to carry AmB, have been brought to clinical trials. However, they have unique specific properties (such as magnetic, optical, redox, etc.) that can be added to those of AmB or beneficially influence those of AmB synergistically. Moreover, there are many reports of the pre-clinical development of such objects as carriers of AmB or for the development other anti-infectious strategies
[16]. Inorganic nanoparticles are structured with a well-organized core made of metal or carbon atoms surrounded by an organic corona (i.e., inorganic/organic core–shell particles). The core can bring, depending on the material it is made with, magnetic or optical properties, while the corona can be functionalized by drugs (e.g., AmB) or other molecules. They represent ideal tools for the targeting and the recognition of the site of action (antibody, aptamer, substrate for an enzyme, ligand for a receptor)
[17]. They can be combined with other properties (i.e., interact with radiation) to succeed in the development of nano-objects dedicated for both therapy and diagnostics (i.e., theranostic)
[17][18][17,18]. These nanoparticles often exhibit redox properties. This is very interesting since it has now also been described that AmB is characterized by both oxidant and reductive activities, usually known as a Janus face. Indeed, Janus was a Roman god depicted with two faces; one looking ahead, the other behind.
2. Inorganic Nanoparticles Carrying Amphotericin B
2.1. The State-of-the-Art of Lipidic Formulations of Amphotericin B on the Market or under Clinical Trials
Due to its chemical structure, AmB is lipophilic, completely insoluble in water, sparingly soluble in alcohol, and highly soluble in dimethylformamide or dimethylsulfoxide
[19][66]. Even though the molecule presents two groups (carboxylic acid and primary amine) associated with ionization constants (pKa), the molecule is globally neutral at physiological pH as it is both positively and negatively charged. AmB is characterized by poor oral permeability, besides a degradation occurring in the stomach. AmB is presented in its classical formulation as micelles of sodium deoxycholate. These parameters may explain why researchers focus on its formulation in so many works. The nanoparticle formulations based on liposomes or lipids increase the therapeutic index of the molecule, decreasing its toxicity, especially nephrotoxicity, while retaining the same efficacy
[20][21][22][67,68,69]. Indeed, lipid formulations of AmB limit nephrotoxicity, but tubule cells remain still vulnerable to some forms of superimposed injury
[23][70]. In 2020, Hnik and coll. tested a single dose of an oral formulation based on liposomal amphotericin (iCo-019) on healthy people. The objective of this study was to develop a molecule that is easy to administer, stable, and non-toxic while maintaining effective pharmacological activity. The data of the randomized controlled trial has demonstrated that the single dose of iCo-019 demonstrated a good tolerance of the molecule and a reduction in its toxicity
[24][71].
These formulations present an innovation, particularly in limiting nephrotoxicity, which explains why they are reserved to treat people suffering from kidney diseases. The products under clinical trial clearly open new opportunities in terms of administration routes. However, from a redox point of view, they do not present any of these properties.
2.2. Inorganic Nanoparticles as Modulator of AmB Redox Properties
2.2.1. Strategies to Functionalize Inorganic Nanoparticles with Amphotericin B
Numerous nanoparticles were synthesized and functionalized to obtain particles carrying AmB.
The nanoparticles were made of a metal or metallic oxide (e.g., silver, gold, iron, and zinc), or they were based on carbon (with carbon quantum dots, graphene, nanotubes) or on calcium phosphate, or on layered double hydroxides, or on silica, or even based on core–shell particles (Pd@Ag nanoparticles)
[25][26][27][28][75,83,100,101]. The synthesis of nanoparticles was realized mainly via the bottom-up approach (using building blocks that further organize in nanoparticles upon a trigger, e.g., reduction, irradiation, etc.). A majority of researchers used chemical processes, while some research described the production of nanoparticles (Ag, Au and iron oxide) via different methods: phytosynthesis using extracts of
Isatis tinctoria,
Maytenus royleanus [25][75],
Cucumis melo L var makuwa,
Prunus persica L.
[29][85], using Chinese cabbage or maize silky hair
[30][102]; or using a green synthesis by
Punica granatum [31][103]; or by biosynthesis using
Acidophilic Acinetobacter P. columellifera subsp.
Pallida [32][76]; or 14
Acinetobacter spp. isolates
[33][77]. In addition, two studies used AmB to directly reduce the Ag
+ into Ag
0 or Au
3+ into Au
0 with success, highlighting the antioxidant character of AmB
[26][34][78,83]. The strategies used to obtain nanoparticles carrying AmB are illustrated in
Figure 2.
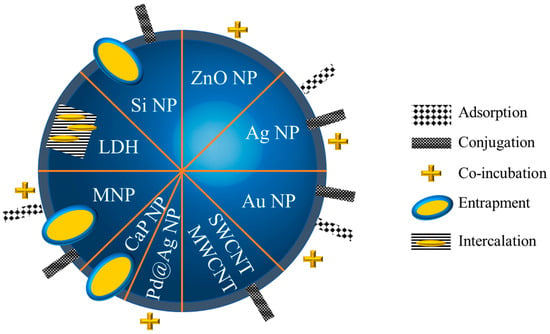
Figure 2. Different strategies employed to carry AmB. NP: nanoparticles; LDH: layered double hydroxide; MNP: magnetic nanoparticles; CaPNP: calcium phosphate nanoparticles; SWCNT: single-walled carbon nanotubes; MWCNT: multi-walled carbon nanotubes.
Common strategies were developed to carry the drug: they rely on adsorption, i.e., a weak interaction between the silver core and mycosamine group or polyol group
[34][35][78,104] or between the nanoparticle and AmB; or conjugation, realized by a strong interaction, e.g., covalence with the use of a spacer
[36][37][84,88]; or entrapment or intercalation between layers
[38][105] within the nanoparticle and the simple co-incubation of nanoparticles and AmB.
In some studies, the authors took advantage of the unique properties of the inorganic nanoparticles, besides their capacity to modulate the redox signaling of the organisms. For example, Ahmad and coll. demonstrated an increase in the activity of their silver nanoparticles carrying AmB upon UV irradiation
[39][73]. AgNP are particularly studied because they have also been known for years for their anti-infectious activity as explained above. In another study, carbon quantum dots were functionalized by AmB and used as a new method for the specific detection of
C. albicans for diagnostic purposes
[40][106]. Iron oxide nanoparticles are also interesting due to their response to a magnetic field that can induce the generation of controlled non-invasive heat and efficient drug delivery at the selected site
[29][85]. Various designs of iron oxide nanoparticles (34–40 nm) coated with bovine serum albumin and targeted with AmB (AmB-IONP), were formulated via a layer-by-layer approach, and tested for their antifungal activity. These compounds showed improved antifungal activity efficacy against
C. albicans and
C. glabrata clinical isolates
[41][97].
2.2.2. Inorganic Nanoparticles as Synergic Prooxidants
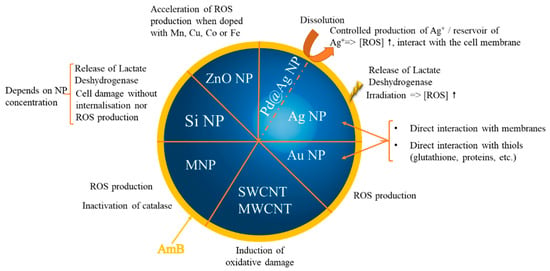
Among the published articles, a lot of studies highlight the combined or synergistic redox properties of nanoparticles carrying AmB. Only a few papers concentrated on their activity against pathogens without exploring the involved redox mechanisms. The proposed redox mechanisms are represented in Figure 3. One can easily understand that oxidative stress can be generated by nanoparticles and/or AmB and then self-sustained. It is very difficult to determine the first actor due to the tight interconnectivity of the mechanisms.
Figure 3. Main mechanisms implied in the prooxidative effect of nanoparticles carrying AmB at the origin of the described synergistic activity. NP: nanoparticles; MNP: magnetic nanoparticles; SWCNT: single-walled carbon nanotubes; MWCNT: multi-walled carbon nanotubes; ROS: reactive oxygen species.
A synergistic effect was almost always highlighted when an oxidative stress was either demonstrated or hypothesized. The effect is therefore superior to the one induced by nanoparticles or AmB alone. Recently, the same phenomenon was observed with AmB and gentamicin-loaded nanosheets/nanoneedles-based boron nitride films
[42][107]. These films exerted an anti-infectious activity against
Neurospora crassa and antibiotic-resistant
E. coli. Another study using molecules other than AmB also showed the synergic effect of nanoparticles carrying antibiotics explained by oxidative stress, for example, silver nanoparticles combined with ampicillin, chloramphenicol, and kanamycin
[43][108] or with neomycin or gentamicin
[44][109]. However, besides their redox properties, nanoparticles possess other advantages, since they can pass through physiological barriers and penetrate more easily into pathogens due to their small size
[33][45][77,110]. After entering into the cells, the nanoparticles disrupt the membrane integrity which creates a passage for drugs across the cell membrane, improving their action at the target site. This was shown for silver nanoparticles
[33][77]. Amphotericin B-silver hybrid nanoparticles (AmB-Ag) have been reported to be a highly effective form of this antibiotic to combat fungi. In a study analyzing the interaction of AmB-Ag with
C. albicans cells using molecular spectroscopy and imaging techniques, the antifungal activity of the nanocomplex system of the disintegration of the cell membrane was demonstrated, which occurs within a few minutes of treatment. This activity increases considerably when the treatment is in the form of hybrid silver nanoparticles. Experimental results show that AmB-Ag can effectively cross the cell wall barrier and deliver antibiotic molecules to cell membranes, thus activating oxidative stress
[46][111].
Nevertheless, this prooxidant effect was sometimes the origin of a toxicity
[47][112]. Researchers have demonstrated that the toxicity of silica nanoparticles carrying AmB was more important than that of the unloaded silica nanoparticles on human fibroblasts and on human endothelial cells. Moreover, the same authors have demonstrated that amphotericin B-functionalized SiO
2 NPs with an average size of 5 and 80 nm have antifungal activity against several strains of
Candida species
[48][113]. This effectiveness was also demonstrated when SiO
2 NPs were immobilized using amphotericin B in the case of dental resins
[49][114]. In another study, AmB macrocyclic polyene was used as a reducing agent and stabilizing agent during the manufacture of Ag NPs. AmB-Ag nanoparticles (with an average size of 4 nm) have an inhibitory effect on the growth of
Aspergillus niger,
Candida albicans, and
Fusarium culmorum. The authors attributed the high antifungal effectiveness of AmB-Ag NPs to the synergistic effect between AmB and Ag
+ ions
[34][78].
ZnO-PEGylated AMB (ZnO-AmB-PEG) nanoparticles demonstrated their antifungal effects on two strains of
Candida spp. When comparing the results obtained by treatment with ZnO-AmB NPs and free AMB against
C. albicans and
C. neoformans, it was determined that ZnO-AmB-PEG NPs significantly reduced the growth of fungi. Additionally, the toxicity was studied using in vitro blood hemolysis, in vivo nephrotoxicity. ZnO-AmB-PEG significantly reduced leukocyte counts, creatinine, and blood urea nitrogen levels, compared to AmB. The authors suggested that ZnO-AmB-PEG could be tested and used clinically
[50][115]. On the contrary, other works reported an absence of toxicity on the kidneys, liver, and spleen of Golden Syrian hamsters
[51][87], Swiss mice
[52][91] and Balb/c mice
[53][95] as well as on red blood cells
[54][79]. In the latter, this was explained by the association of the functionalized nanoparticles with the circulating high-density (HDL) and low-density lipoproteins (LDL). Toxicity issues related to inorganic nanoparticles are a long-running story. Among others, the physicochemical parameters of nanoparticles, the material they are made with, and their possible degradation products are key points to understand since they may explain the observed phenomenon. It remains very difficult to express general rules about this toxicity
[55][56][61,116].
2.2.3. Inorganic Nanoparticles as Synergic Antioxidants
Two publications focused on the antioxidant activity of nanoparticles carrying AmB
[29][30][85,102]. In both, nanoparticles (made either of magnetite iron oxide or of gold) were synthesized using plants: either the silky hair of corns or the outer leaves of Chinese cabbage or other aqueous extracts of outer oriental melon peel and peach. It is likely that the nanoparticle corona contained antioxidant biomolecules such as flavonoids and polyphenols besides the activity of the metallic core of the nanoparticles. In the two works, the authors highlighted a strong antioxidant property due to the scavenging of radicals (i.e., 1,1-diphenyl-2-picrylhydrazyl, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and nitric oxide) and also a strong proteasome inhibition. It has already been described that the antioxidant activity coming from the inorganic core of nanoparticles can be enhanced when functionalized by other antioxidants such as reduced glutathione
[57][117]. These nanoparticles, when combined with AmB, proved to have synergic activity against
Candida spp. The level of antioxidant property was correlated to the antifungal activity.
The synergic antioxidant effect is less studied in the literature. The obtained antioxidant effect may be linked to the corona of such nanoparticles that are based on extracts of plants, which can bring an antioxidant activity by themselves. The synergistic aspect of the nanoparticle combined with AmB is not totally obvious in these examples. Other studies will certainly bring more robustness to this activity in the future.