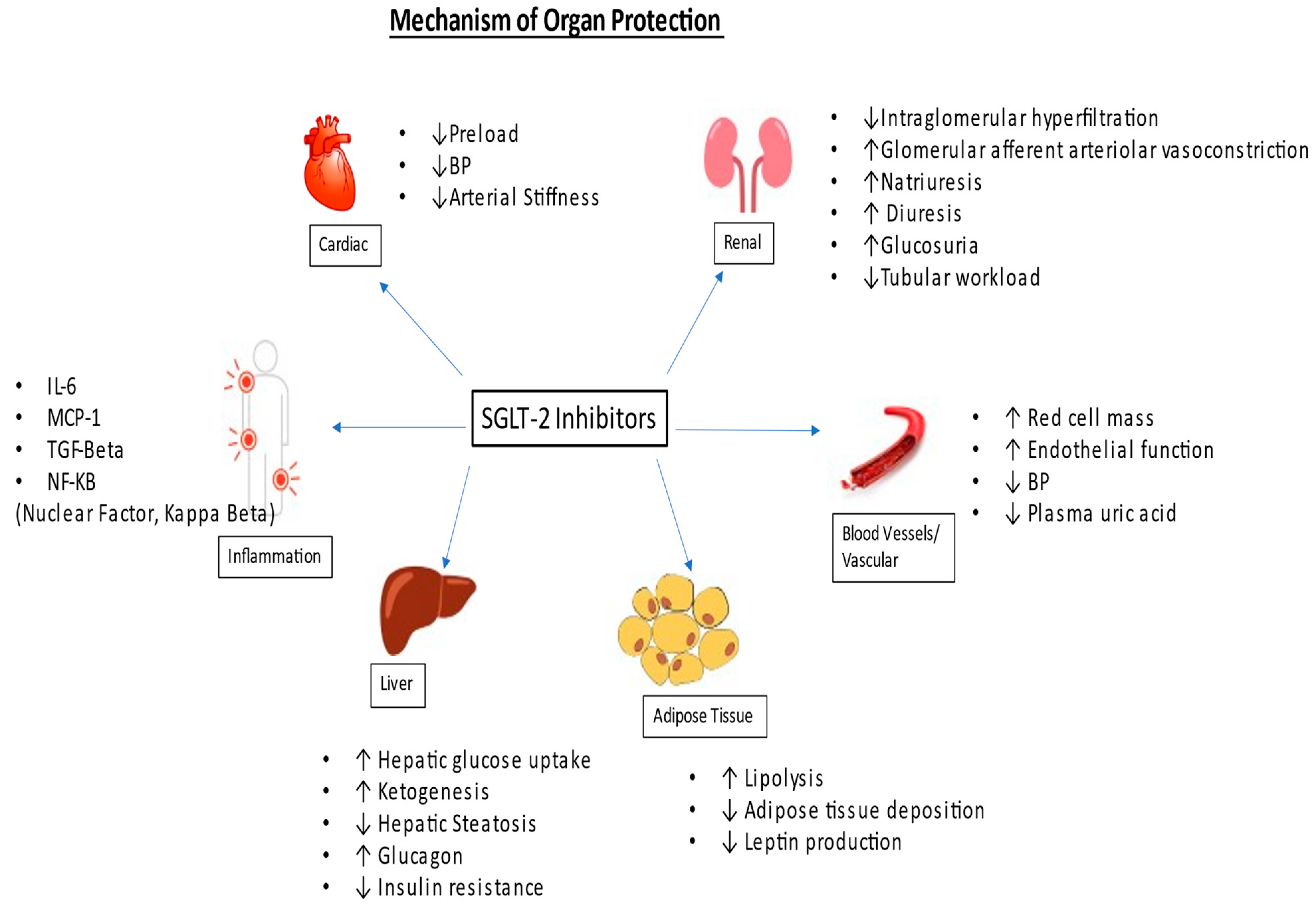
Diabetic kidney disease (DKD) causes a progressive decline in renal function, leading to end-stage kidney disease (ESKD), and increases the likelihood of cardiovascular events and mortality. The introduction of the sodium-glucose co-transporter 2 (SGLT-2) inhibitor has been a game changer in managing chronic kidney disease (CKD) and congestive heart failure (CHF). These agents not only slow down the progression of kidney disease but also have cardioprotective benefits, including for patients with congestive heart failure and atherosclerotic cardiovascular disease. Some evidence suggests that they can decrease the risk of stroke as well.
- diabetic kidney disease
- nephropathy
- SGLT-2 inhibitor
- stroke
- CHF
- MRA
- flozins
1. Introduction
2. Mechanism of Action
3. Effectiveness
3.1. Effectiveness in CKD
|
Trial |
CREDENCE |
DAPA-HF |
DAPA CKD |
VERTIS |
EMPA KIDNEY |
|---|---|---|---|---|---|
|
Drug dose |
Canagliflozin 100 mg 100 mg |
Dapagliflozin 10 mg |
Dapagliflozin 10 mg |
Ertugliflozin 5, 15 mg |
Empagliflozin 10 mg |
|
Year published |
June 2019 |
November 2019 |
March 2020 |
June 2020 |
November 2022 |
|
Median follow up |
2.6 yrs. |
18.2 months |
2.4 yrs. |
3.5 yrs. |
2 yrs. |
|
eGFR on enrollment |
>30, UACR 300–5000 mg/gm |
>30 (40% pt had eGFR < 60) |
25–75, UACR 200–5000 mg/gm |
>30 |
20–45 or >45 < 90, if UACR > 200 mg/gm |
|
DM-2 on enrollment |
DM-2 only |
42% had DM-2 |
30% non-diabetic |
DM only |
Both Diabetic and non-Diabetic 2.2% had DM-type 1 |
|
CVD on enrollment |
50% participants |
All participants |
37% participants |
76.3% had CAD, 23% had CHF |
26% participants |
|
Primary outcome |
ESKD, doubling of serum creatinine, death from renal or CV causes. |
CV death, heart failure hospitalization, all-cause mortality |
ESKD, >50% drop in eGFR, death from renal and CV causes |
MACE (CV death, MI, stroke) |
ESKD, sustained decrease in eGFR < 10, in eGFR > 40%, death due to renal or cv Causes. |
|
Secondary outcome |
Cardiovascular mortality, all-cause mortality, MI, stroke. |
ESKD, >50% drop in eGFR lasting >28 days, death from renal causes. |
Same as above. |
Composite of renal death, dialysis, doubling of serum creatinine |
Heart failure hospitalization, all cause mortality, all cause hospitalization |
|
Results |
Reduction in primary outcome by 30–35% |
Reduction in - CV death, HF by 24%, - All cause mortality by 17% |
Reduction in primary outcome by 35–45% |
no statistically significant improvement in primary outcomes. |
Reduction in primary outcome by 25–30% |
|
NNT (for primary outcome) |
22 |
21 |
19 |
Not available |
Not available |
DM-2—Type 2 Diabetes mellitus; CAD—coronary artery disease; CHF—congestive heart failure; CVD—cardiovascular disease; ESKD—end-stage kidney disease; MI—myocardial infarction; NNT—number needed to treat; UACR—urinary albumin-to-creatinine ratio.
3.2. Effectiveness in CHF
3.3. Effectiveness in Stroke
References
- USRDS. Annual Data Report | USRDS. Available online: https://usrds-adr.niddk.nih.gov/2022 (accessed on 18 July 2023).
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B. IDF Diabetes Atlas: Global, regional, and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119.
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28.
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P. The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy. N. Engl. J. Med. 1993, 329, 1456–1462.
- Brenner, B.M.; Cooper, M.E.; De Zeeuw, D. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869.
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 851–860.
- Investigators, D. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357.
- Heerspink, H.J.L.; Kosiborod, M.; Inzucchi, S.E.; Cherney, D.Z. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018, 94, 26–39.
- Packer, M. Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics. Am. J. Kidney Dis. 2021, 77, 280–286.
- Fine, L.G.; Norman, J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 2008, 74, 867–872.
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166.
- Dekkers, C.C.J.; Petrykiv, S.; Laverman, G.D.; Cherney, D.Z.; Gansevoort, R.T.; Heerspink, H.J.L. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes. Metab. 2018, 20, 1988–1993.
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Har, R.; Fagan, N.; Johansen, O.; Woerle, H.-J.; von Eynatten, M.; Broedl, U.C. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc. Diabetol. 2013, 13, 28.
- Mazer, C.D.; Hare, G.M.; Connelly, P.W.; Gilbert, R.E.; Shehata, N.; Quan, A.; Teoh, H.; Leiter, L.A.; Zinman, B.; Jüni, P.; et al. Effect of Empagliflozin on Erythropoietin Levels, Iron Stores, and Red Blood Cell Morphology in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease. Circulation 2020, 141, 704–707.
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306.
- Bakris, G.; Oshima, M.; Mahaffey, K.W.; Agarwal, R.; Cannon, C.P.; Capuano, G.; Charytan, D.M.; De Zeeuw, D.; Edwards, R.; Greene, T.; et al. Effects of Canagliflozin in Patients with Baseline eGFR <30 mL/min per 1.73 m2. Clin. J. Am. Soc. Nephrol. 2020, 15, 1705–1714.
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446.
- Heerspink, H.J.L.; Jongs, N.; Chertow, G.M.; Langkilde, A.M.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; Stefansson, B.V.; Toto, R.D.; et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 743–754.
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127.
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008.
- Inzucchi, S.E.; Docherty, K.F.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Solomon, S.D.; Verma, S.; Bělohlávek, J.; et al. Dapagliflozin and the Incidence of Type 2 Diabetes in Patients With Heart Failure and Reduced Ejection Fraction: An Exploratory Analysis From DAPA-HF. Diabetes Care 2020, 44, 586–594.
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424.
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461.
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: Rationale and design of the DELIVER trial. Eur. J. Heart Fail. 2021, 23, 1217–1225.
- Kelly, D.; Rothwell, P.M. Impact of multimorbidity on risk and outcome of stroke: Lessons from chronic kidney disease. Int. J. Stroke 2020, 16, 758–770.
- Zelniker, T.A.; Bonaca, M.P.; Furtado, R.H.; Mosenzon, O.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.; et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients with Type 2 Diabetes Mellitus. Circulation 2020, 141, 1227–1234.
- Ong, H.T.; Teo, Y.N.; Syn, N.L.; Wee, C.F.; Leong, S.; Yip, A.S.Y.; See, R.M.; Ting, A.Z.H.; Chia, A.Z.; Cheong, A.J.Y.; et al. Effects of Sodium/Glucose Cotransporter Inhibitors on Atrial Fibrillation and Stroke: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2021, 31, 106159.
- Zhou, Z.; Jardine, M.J.; Li, Q.; Neuen, B.L.; Cannon, C.P.; de Zeeuw, D.; Edwards, R.; Levin, A.; Mahaffey, K.W.; Perkovic, V.; et al. Effect of SGLT2 Inhibitors on Stroke and Atrial Fibrillation in Diabetic Kidney Disease. Stroke 2021, 52, 1545–1556.
- Chang, S.; Chen, J.; Huang, P.; Wu, C.; Wang, Y.; Hwang, J.; Tsai, C. Sodium-Glucose Cotransporter-2 Inhibitor Prevents Stroke in Patients With Diabetes and Atrial Fibrillation. J. Am. Heart Assoc. 2023, 12, e027764.