Muscle growth, or muscle hypertrophy, is a complex process regulated by several molecular pathways. The IGF-1/PI3K/Akt/mTOR pathway is a vital signaling cascade in muscle growth that involves various interconnected mechanisms. Its activation increases protein synthesis, reduces protein degradation, and improves cell growth. Akt activation is crucial in promoting muscle protein synthesis in response to exercise and nutrient intake in young individuals. The timing of exercise and protein intake also affect Akt activation and subsequent muscle protein synthesis. While exercise alone did not increase Akt and mTOR phosphorylation, protein ingestion afterward did so in a dose-dependent manner. Growth hormone (GH) promotes the uptake of essential nutrients, such as glucose and amino acids, into muscle cells for energy production and protein synthesis. Testosterone is one of the most potent naturally secreted androgenic-anabolic hormones, and its biological effects include promoting muscle growth.
- exercise
- nutrition
- skeletal muscle
1. The Molecular Mechanisms behind Muscles Growth in Young Subjects: Exercise, Nutrients, and Hormones
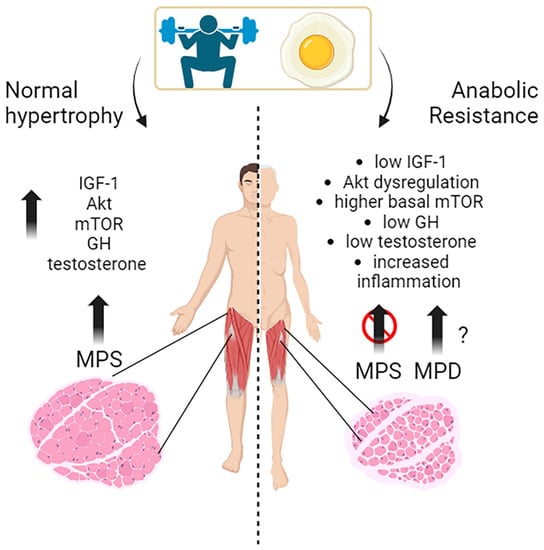
2. Effects of Substrates and Exercise on Skeletal Muscle Protein Synthesis in Young, Middle-Aged Subjects
Accretion of muscle mass depends on physiological, metabolic, and hormonal factors, as well as on physical activity. Conversely, it is hindered by inactivity, malnutrition, overt diseases, and/or subtle, chronic pathological conditions [19][20][21][22][23][24][31,32,33,34,35,36]. The main protein-anabolic factors, at both the whole-body and skeletal muscle level, are the proteins and/or the amino acids themselves (i.e., the protein building blocks), as well as physical activity/exercise. In addition, energy availability, anabolic hormones (insulin, human Growth Hormone (hGH), IGF-1, β-agonists, anabolic steroids, and see also above), adequate tissue perfusion, and, in general, a “healthy status” (i.e., the absence of both overt diseases and subtle pathological conditions, such as a chronic sub-inflammatory status) also condition skeletal muscle accretion. Furthermore, the effects of any of these factors could be divided into either “acute” (i.e., detectable under acute experimental conditions) or “chronic” (i.e., following repeated stimuli, with end-point results tested sometime after). Protein anabolism is thus achieved through the stimulation of protein synthesis and/or the inhibition of protein degradation. MPS can be determined in vivo either by measuring the incorporation of infused amino acid stable isotopes into muscle by biopsy (Figure 2) and/or through measurements of the A-V difference in labeled as well as unlabeled amino acid(s) across a sampled district predominantly constituted by skeletal muscle, typically a limb (either the leg or the forearm) [25][37] (Figure 3).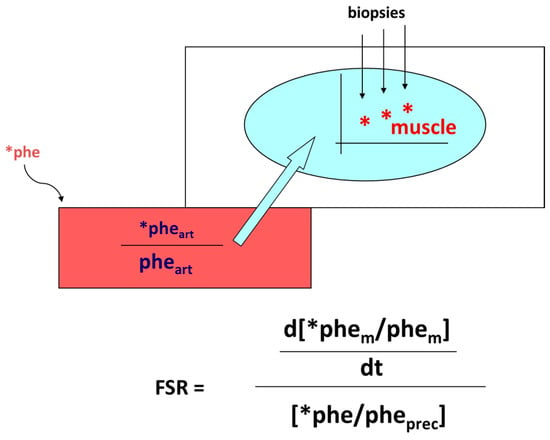
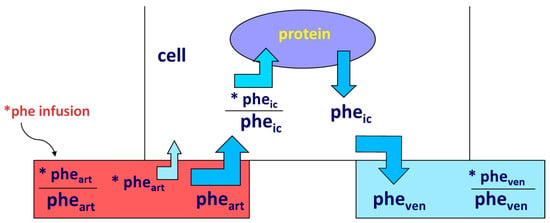
2.1. Effects of Proteins and Amino Acids
2.2. Effects of Exercise and Nutrition on Muscle Protein Synthesis and Accretion
Exercise is a potent stimulator of muscle protein synthesis, particularly in the recovery phase [49][79], and it positively interacts with protein/amino acid ingestion in the stimulation of skeletal muscle anabolism. Muscle mass accretion following resistance exercise combined with food ingestion is observed even following an adequate habitual protein intake (≥0.8 g kg−1 day−1) [50][81]. A greater protein intake (1.8–3.0 g kg−1 day−1) further augments lean body mass (i.e., protein) accretion without increasing fat mass, when compared to an energy-rich, low-protein intake (≈5% of energy as protein) [50][81]. The high-quality whey protein, combined with resistance exercise in young adults, exerted a greater effect on MPS than equivalent doses of lower-quality proteins, such as soy protein or casein, an effect, however, still present up to 3–5 h post-exercise [38][55]. As reported above, a 20 g dose of whey protein was sufficient for the maximal stimulation of post-absorptive myofibrillar MPS in young men, whether or not exercising [37][54], and it was effective up to 3–5 h after exercise [28][51][40,82]. Conversely, others reported that the response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g rather than 20 g of ingested whey protein [52][71]. After the ingestion of incremental doses of mixed milk protein (0, 15, 30, or 45 g) together with 45 g carbohydrate, the 30 g protein dose was sufficient to maximize the myosin synthesis rates during recovery from a single bout of endurance exercise in young men [53][74]. The intake of another high-quality protein, i.e., egg protein, as low as 5–10 g (approximating that contained in a single ≈ 60 g egg: ≈ 6.8 g total protein), increased MPS above basal following resistance exercise, reaching a maximum with a dose of 20 g egg protein [54][72].2.3. Effect of Other Substrates
Protein synthesis is an energy-requiring process [55][86]; therefore, energy-providing substrates such as glucose and fat may affect protein turnover too. The activities of the cellular pathways controlling protein turnover are bio-energetically expensive and therefore depend on intracellular energy availability (i.e., macronutrient intake) [56][87].Glucose
Glucose increased muscle protein synthesis in vitro [57][88]. Testing glucose-induced or derived substrates separately, tissue ATP decreased during incubation with lactate, and lactate + pyruvate supported protein synthesis better than pyruvate or glucose. The data on the effects in humans of either glucose or fat on protein metabolism, specifically on skeletal muscle, are scarce, complex, and not univocal [30][42]. Enteral glucose administration did not affect either duodenal mucosal protein FSR or the activities of mucosal proteases [58][89]. An oral glucose load, and the simultaneous glucose-induced stimulation of insulin secretion, did not alter the rate of whole-body protein synthesis or breakdown [59][90].Lipids and Ketones
The effects of either lipid or ketone infusion/administration in humans are complex. Lipid infusion in humans did not affect proteolysis [60][96]. In contrast, medium-chain fatty acid infusion apparently increased leucine oxidation and, therefore, net protein catabolism [61][97]. Thus, the effects on whole-body protein degradation may depend on the fatty acid length [62][98]. The increase in FFA decreased basal muscle protein synthesis, but not the anabolic effect of leucine [63][99]. When associated with dietary protein ingestion, neither acute nor short-term dietary fat overload impaired skeletal MPS in overweight/obese men in the post-prandial phase, thus excluding a role by dietary accumulation of intramuscular lipids on the anabolic response to meal ingestion [64][100]. The infusion of 3OHButyrate decreased both whole body and forearm protein turnover (measured by phenylalanine/tyrosine tracers), as well as phenylalanine catabolism, in post-absorptive conditions, whereas it did not modify the insulin-induced effects following an euglycemic clamp [60][96].Other Nutritional Interventions
β-alanine supplementation may increase physical performance in middle-aged individuals [65][104] and improve physical performance during exercise [66][105]. Creatine may increase muscle mass in combination with resistance exercise, although the mechanism(s) of action remain elusive. Short-term creatine monohydrate (CrM) supplementation may exert anti-catabolic actions on selected proteins in men, but it did not enhance either whole-body or mixed-muscle protein synthesis [67][106]. Acute metabolic studies testing various substances may provide useful information for estimating the efficacy of potentially anabolic agents [68][107].3. Hormones and Related Drug Interventions
3.1. Insulin
Although insulin has an undisputed anabolic effect on tissue protein, its experimental demonstration in vivo has challenged the investigators over time, mainly because its administration induces a decrease in amino acid plasma concentration, thus possibly obscuring its direct effect in muscle. A major advancement was the maintenance of the “amino acid “clamp” at baseline during insulin infusion or injection, also achieved when insulin was directly infused into the artery perfusing a muscle-rich organ (such as the leg or the forearm), thus avoiding a system perturbation of the aminoacidemia and allowing, at the same time, the insulin effect in the perfused limb to be studied selectively, using any of these techniques. Muscle protein synthesis and degradation were determined by combining amino acid isotope infusion with arterial–venous limb catheterization, often complemented by muscle biopsy in some studies.3.2. Glucocorticoids
Cortisol, often referred to as a stress hormone, has both catabolic and anabolic effects, depending on the context. Cortisol-induced secondary sarcopenia (i.e., specifically induced either by either an exogenous administration or an endocrine disease) is a frequent finding in the clinical setting. Chronic glucocorticoid exposure induces loss of lean body mass by decreasing protein synthesis and increasing degradation [69][70][71][111,112,113]. The protein-catabolic effect of prednisone is antagonized by growth hormone [70][112]. In humans, the administration of 8 mg dexamethasone daily for 4 days antagonized the anti-proteolytic effect of insulin in the forearm [72][114].3.3. Human Growth Hormone (hGH) and IGF-1
In humans, hGH administration increased whole-body [70][112] and muscle protein synthesis [73][115]. The insulin-like growth factor-binding proteins (IGFBPs) bind to IGFs, modulating their activity and availability. They help in regulating IGFs’ actions in various tissues, including muscle. When rhIGF-I was infused at a rate achieving plasma IGF-I concentrations close to those observed following rhGH treatment, and yet avoiding the IGF-1-induced hypoglycemia, proteolysis and protein synthesis were not affected, even in the presence of prednisone treatment [74][116]. However, when rhGH and rhIGF-I were administered simultaneously, nitrogen balance was remarkably improved [74][116]. IGF-1 exhibits several splicing variants, IGF-1Ea, which is a circulating factor synthesized in the liver, and IGF-1Eb and IGF-1Ec, recognized as Mechano-Growth Factors (MGFs), which manifest in skeletal muscles of rodents and humans, respectively [75][117]. The act of stretching or overloading skeletal muscles triggers a rise in IGF-1 mRNA, particularly emphasizing the specific IGF-1Ec variant (MGF) [75][117]. The extent to which IGF-1 alternative splicing variants might induce greater hypertrophy compared to IGF-1 per se remains partially unresolved [76][118]. Notably, the effects of the synthetic E-domain peptide mimetic have been elucidated: the MGF-24aa-E peptide (YQPPSTNKNTKSQRRKGSTFEEHK) activates satellite cells, prompting their replication [75][117].3.4. Catecholamines
Epinephrine has both an α- and β-receptor affinity. Epinephrine infusion in humans depressed plasma amino acid concentrations, particularly the essential ones, however without changing leucine or phenylalanine flux [77][120], nor did it impair the disposal of exogenous amino acids in humans [78][121]. However, in another study, the increase in plasma epinephrine concentrations inhibited proteolysis and leucine oxidation in humans via beta-adrenergic mechanism, compatible with an anabolic effect [79][122].3.5. Estrogens
Although estrogens are female sex hormones, they are also present in smaller amounts in males. Estrogen plays a role in maintaining bone health, promoting protein synthesis and muscle growth, and influencing body composition, in both males and females. While the exact mechanisms by which estrogens affect muscle growth are still being studied, research suggests that they can, directly and indirectly, affect muscle tissue. One way estrogens may influence muscle growth is by promoting protein synthesis, interacting with receptors in muscle cells, and activating signaling pathways involved in protein synthesis [80][126]. Decreased estrogen-associated signaling impairs mitochondrial function leading to muscle atrophy [81][127]. Estrogens can also indirectly affect muscle growth by modulating the production and activity of other hormones, such as growth hormone and insulin-like growth factor 1 (IGF-1), which are important for muscle development. Estrogens may regulate the release of these hormones from the pituitary gland and the liver, thereby influencing muscle growth [82][128].3.6. Androgens
Testosterone is a potent anabolic stimulus primarily through improvement in the re-utilization of amino acids released from protein degradation [83][132] (see also the above paragraph), and this will be further discussed below. Testosterone and progesterone, but not estradiol, stimulated muscle protein synthesis in postmenopausal women [84][133].
