Many algae respond to the 许多藻类对一氧化碳有反应2通过诱导一氧化碳限制海水2浓缩机理(CO2 limitation in seawater by inducing a CO2 concentrating mechanism (M)以获得足够的无机碳来满足其光合作用需求。为了评估不同藻类中CCM) to obtain sufficient inorganic carbon to meet their photosynthetic needs. To assess the diversity of the 功能和活性的多样性,需要建立可靠的指标来测量和量化CCM functions and activities in different algae, reliable metrics to measure and quantify the relative functions of CCM need to be established.的相对功能。正如Badger等人[47]所报道的那样,我们筛选出几种可能适合测量大型藻类CCM的指标。
- CO2 concentrating mechanism
- Ulva sp.
- green tide
- inorganic carbon transporters
- carbonic anhydrase
- Rubisco
1. CO Affinity of Photosynthesis Versus Rubisco
1. CO2 Affinity of Photosynthesis versus Rubisco
2
2
2
m
2
2
3 species might require a CCM [1]. However, due to the limitation of extraction technology, the affinity determination of photosynthetic organism Rubisco lacked accuracy. With the improvement of extraction and analysis techniques, this method has become widely used to evaluate algal CCMs. In general, the researchers measured the photosynthetic oxygen evolution rate under different inorganic carbon concentrations, and used the data analysis software to fit the inorganic carbon response curve (P-C curve) by using Michaelis-Menten equation, where K
m
2
m
2
2
2
2 [2]. For example, C. reinhardtii’s ratio of K
m
2
m
2) is approximately 1:30, and that of Ulva sp. is about 5–10:68 [3][4][5], both apparently rely on CCM to improve the efficiency of Rubisco, whereas other algae with close specific gravity may not use CCM.
2. Effects of CA Inhibitors
Because CA is central in all chloroplast CCM models, determining the effect of CA inhibitors is useful in studying the functional aspects of algal CCM. This is especially true for chloroplast-penetrating inhibitors like EZA (Ethoxzolamide), which are likely to inhibit most forms of internal CA. EZA is often used to obtain low inorganic carbon affinity for photosynthesis of algae. If the algae showed low inorganic carbon affinity under the action of EZA, it is proved that there is intracellular CA-mediated CCM in the algae, but no effect may not be the conclusive evidence of CCM deletion. For CA inhibitors that cannot penetrate cell membranes, such as AZA (Acetazolamide), only apply to inhibit the activity of external CA [6], the effect on the function of chloroplast CCM is less easily explained. By comparing the relative effects of EZA and AZA, the relative contributions of internal and external CA forms to photosynthetic Ci absorption can be determined (
Figure 1). For example, in the study of Gao et al. [7], after adding AZA the net photosynthetic rate of U. linza decreased, and the inhibition rate was noted to be 26.26%. Compared with AZA, EZA demonstrated a higher inhibition rate of photosynthesis (75.19%), which indicated that the internal CA contributed more to the absorption of photosynthesis. Xu et al. [8] found that U. linza and U. prolifera have obvious extracellular and intracellular CA activity by using different CA inhibitors. However, extracellular CA enzyme activity itself accelerates the mutual conversion between HCO
3−
2, but cannot affect the CO
2 equilibrium concentration and CO
2
3−
2
3−
3−
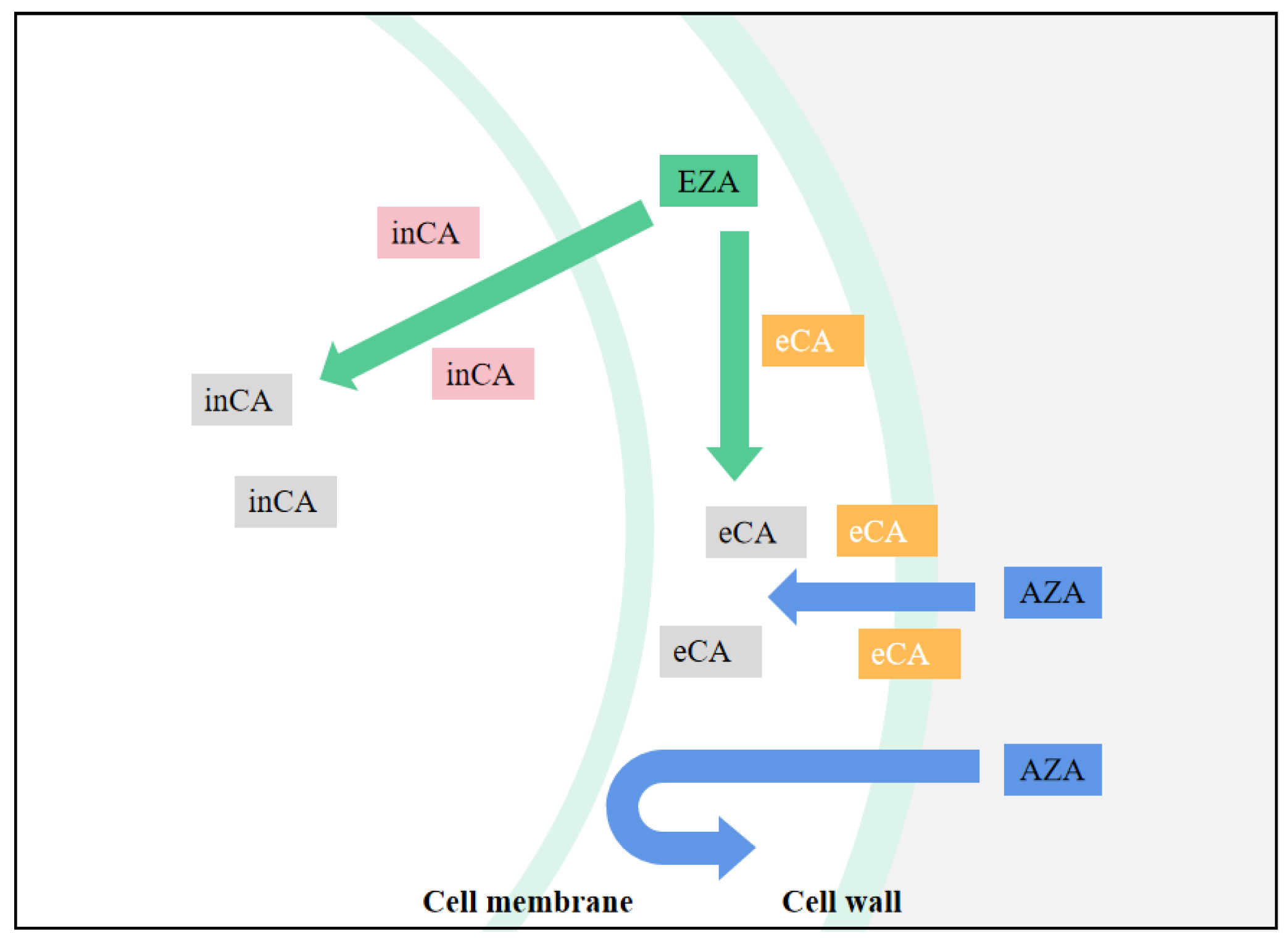
Figure 1
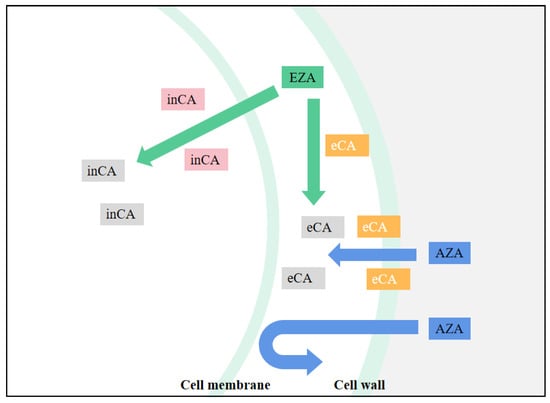
Schematic diagram of CA inhibitor actions. inCA means intracellular CA, eCA means extracellular CA, and when the CA icon is gray, CA is suppressed.
3. Using HCO as Photosynthetic Substrate
3. 使用HCO3−作为光合底物
The function of HCO
3− in photosynthesis has been perceived as a property of algae [9]. The utilization of HCO
3− by algae depends on the involvement of external CA and plasma membrane transporters [10][11][12]. The use of HCO
3− is strongly correlated with the presence of a CCM. However, some macroalgae are not only capable of utilizing HCO
3− as a carbon source, some have demonstrated the use of CO
2 in specific situations. Through the detection of δ
13C isotopes in Gracilaria and Ulva, it was found that when the concentration of environmental CO
2 was high, there was a physiological transition from using HCO
3− almost exclusively to predominantly using CO
2. At the current seawater pCO
2, many macroalgae use HCO
3− rather than dissolved CO
2, and utilize CA to convert HCO
3− to CO
2 for use by Rubisco [13][14][15][16]. For example, Mercado et al. [17] found that under the current seawater CO
2 level, the chlorophyll plants U. rigida and U. compressa cannot obtain sufficient CO
2 through diffusion absorption alone; therefore, a CCM must be used to obtain HCO
3−. However, macroalgae may downregulate their CCM, reduce HCO
3− use, and become dependent on CO
2 as the main carbon source when CO
2 concentrations are high [3][18][19][20]. Consequently, when using these indicators to evaluate the CCM of Ulva sp., there may be a decrease in the use of HCO
3− in certain circumstances, where there is still CCM activity but with a certain degree of downregulation.
4. Changes in Affinity to External Ci Depends on Growth Ci Conditions
4. 与外部Ci的亲和力的变化取决于生长Ci条件
When there is Ci limitation in the external environment, the affinity of the Ci transporter for external CO
2 and HCO
3- increases, and the intracellular and extracellular CA activity also increases. The two work together to increase the affinity of microalgae for external Ci by more than 10 times [10]. This inducible change appears to be strong evidence that cellular infrastructure is involved in supplying CO
2 to Rubisco in chloroplasts These inducible CCM are not limited to microalgae but have been observed in many macroalgae as well, including Ulva [3], Gracilaria [21], Porphyra [22] and Fucus [23]. That is, some algae may have an inducible CCM to meet their environmental needs when facing external Ci periodic constraints, while other algae may not have such a flexible arrangement.
