Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Youssef Youssef and Version 3 by Lindsay Dong.
Uterine Adenomyosis is a benign condition characterized by the presence of endometrium-like epithelial and stromal tissue in the myometrium. Several medical treatments have been proposed, but still, no guidelines directing the management of adenomyosis are available. While a hysterectomy is typically regarded as the definitive treatment for adenomyosis, the scarcity of high-quality data leaves patients desiring fertility with limited conservative options.
- adenomyosis
- medical treatments
- progesterone
- intrauterine devices
- gonadotropin releasing hormones
1. Introduction
Uterine Adenomyosis is a benign condition histologically defined as the presence of endometrium-like epithelial and stromal tissue in the myometrium, along with enlargement of the uterus [1][2][1,2]. Its prevalence is estimated to be around 1%, with an incidence of 29 per 10,000 person–years [3]. Like endometriosis and leiomyoma, adenomyosis is most identified in women aged 41–45 years and is more prominent in the African American population [1][2][3][1,2,3]. The exact etiology of adenomyosis remains unclear, despite multiple proposed theories, such as Müllerian rests, metaplasia of stem cells, genetic mutations, and endometrial invagination into the myometrium [4][5][6][4,5,6]. New theories related to endometriosis pathophysiology could change our current understanding of adenomyosis, as adenomyosis and endometriosis are closely linked, both being estrogen-dependent and often found concomitantly in patients [7][8][9][7,8,9]. One of these newer theories is that genetic-epigenetic changes affect intracellular aromatase activity causing intracellular estrogen production with the subsequent development of inflammatory, fibrotic endometrial-like tissue outside the uterus [10]. It is important to note that although adenomyosis and endometriosis share similar histological features and molecular changes, they differ in pathogenesis, location, and clinical features [11][12][11,12].
Adenomyosis presents clinically with debilitating symptoms such as menorrhagia, chronic pelvic pain, dysmenorrhea, and infertility, requiring treatment [13][16]. Diagnosis of adenomyosis is made via transvaginal ultrasonography (US) or MRI but definitive diagnosis requires histopathological evidence. US findings include heterogeneous myometrium, myometrial cysts, and asymmetric myometrial thickness, in addition to sub-endometrial echogenic linear striations [14][15][16][17][18][17,18,19,20,21]. MRI findings include high-intensity foci representing hemorrhage and increased thickness of the junctional zone representing smooth muscle hyperplasia with accompanying heterotopic endometrial tissue [19][22]. Studies comparing the effectiveness of transvaginal US and MRI have demonstrated the latter to be equal, if not superior, in the diagnosis of adenomyosis [20][21][22][23][24][23,24,25,26,27].
Currently, no guidelines directing the management of adenomyosis are available, despite numerous treatments, both medical and surgical in nature [25][26][28,29]. Although hysterectomy provides a definitive cure, it is not the method of choice for patients willing to preserve future fertility or those who are not medically fit for surgery [27][30]. In light of the increasing trend of late childbearing, various pharmacological therapies and fertility-preserving surgeries have emerged.
2. Medical Therapies for Adenomyosis
2.1. Classical Treatments
3.1. Classical Treatments
23.1.1. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
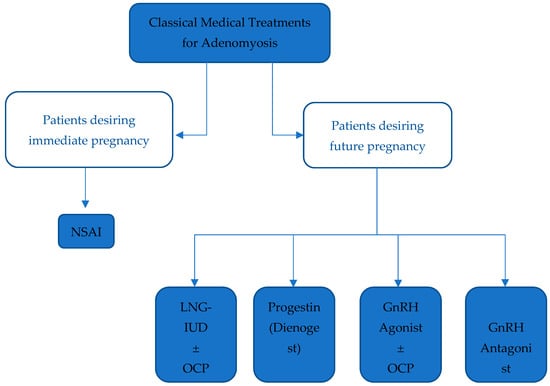
NSAIDs have been proven to be effective in treating dysmenorrhea, despite insufficient evidence to determine the safest and most effective agent in that class [28][33]. A Cochrane database systematic review demonstrated a statistically relevant decrease in heavy menstrual bleeding when comparing NSAIDs to placebo, despite being less effective than hormonal treatments or tranexamic acid [29][34]. Nevertheless, NSAIDs and other analgesics remain the sole treatment option in women with adenomyosis interested in pregnancy [30][35]. In symptomatic women with no interest in conceiving, hormonal treatments are preferred to address chronic abnormal uterine bleeding and pain, with NSAIDS prescribed only in acute exacerbations [30][35].23.1.2. Levonorgestrel-Releasing Intra-Uterine Device (LNG-IUD)
LNG-IUDs diffusing 20 µg/day of levonorgestrel are often used in women with adenomyosis experiencing abnormal uterine bleeding, despite being originally designed for long-term contraception [30][31][35,36]. They exert their control via decidualization and atrophy of the endometrial tissue by creating a hypoestrogenic state and by downregulating estrogen receptors due to high progestin release [32][33][37,38]. Ozdegirmenci et al. found LNG-IUD to be comparable to hysterectomy after measuring hemoglobin levels at 6 months and 1 year of treatment [34][39]. LNG-IUDs are currently the best-evaluated and most efficacious treatment of adenomyosis-related symptoms with a high rate of symptom improvement, minimal sideeffects, and an improvement in the quality of life that is similar to that of a hysterectomy [30][35].23.1.3. Progestins
Progestins exert an anti-proliferative and anti-inflammatory effect, leading to the decidualization and atrophy of endometrial tissue, with a subsequent significant reduction in bleeding [25][28]. Dienogest (DNG) is a synthetic progestogen with properties of 19-norprogesterone and 17α-hydroxyprogesterone derivatives used in the long-term treatment of endometriosis [30][35]. It has been proven to improve primary and secondary dysmenorrhea [35][42]. Despite being used for the treatment of adenomyosis-related symptoms, there is currently no proper therapeutic management protocol for its use in the treatment of adenomyosis [25][28]. Danazol is a synthetic modified progestogen that reversibly inhibits the synthesis of LH and FSH and has a weak androgenic effect [36][45]. It is used in endometriosis to shrink ectopic endometrial tissue in addition to reducing aromatase expression in the eutopic endometrium [37][46].23.1.4. Gonadotropin Releasing Hormone (GnRH) Agonist and Oral Contraceptive Pills (OCP)
GnRH is a decapeptide secreted by the hypothalamic neurons that acts on receptors in the anterior pituitary gland. Continuous prolonged stimulation by GnRH agonists leads to a central downregulation with the suppression of gonadotropin secretion, ultimately leading to the induction of a hypoestrogenic state in addition to an antiproliferative effect within the myometrium [38][51]. Additionally, combined oral contraceptive pills were found to be effective in treating dysmenorrhea [39][52]. Both OCPs and GnRH agonists are used as suppressive hormonal therapies to induce the regression of adenomyosis and improve the severity of symptoms [40][53]. GnRH agonists are also indicated to improve the chances of pregnancy in women with adenomyosis [41][56]. The highest pregnancy rate has been reported in women undergoing frozen embryo transfer after GnRH agonist treatment [42][57]. It is important to note, however, that the use of GnRH agonists for pain and bleeding should be restricted to short-term only due to possible menopausal effects [43][58].23.1.5. GnRH Antagonists
GnRH antagonists are peptide compounds that share a structure like that of natural GnRH and have an immediate antagonist effect on GnRH receptors in the pituitary gland, inhibiting gonadotropin secretion [30][35]. A case report by Donnez et al. describes a patient who, after failing a course of ulipristal acetate, a Selective Progesterone Receptor Modulator (SPRM), was prescribed Linzagolix, a GnRH antagonist. Treatment with Linzagolix reduced adenomyotic lesion size and dysmenorrhea burden, ultimately leading to improved quality of life [44][59]. Another GnRH antagonist, Elagolix, is currently being developed for the long-term treatment of endometriosis and uterine leiomyomas [45][46][47][48][55,60,61,62]. Elagolix has been shown to regress the size of fundal adenomyoma, with an improvement in clinical symptoms and the resolution of pelvic pain [49][63]. The main advantage of GnRH antagonists over GnRH agonists is their ability to maintain sufficient estradiol levels to avoid bone demineralization and estrogen deprivation symptoms [50][64] (Figure 12).
Figure 12. Summarizing classical medical treatments for Adenomyosis. Legend: NSAID: Non-steroidal anti-inflammatory drug, LNG-IUD: Levonorgestrel-releasing intrauterine device, OCP: Oral contraceptive pill, GnRH: Gonadotropin-releasing hormone.
