You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Mónica L Fanarraga.
AB toxins exhibit a protein structure, consisting of two distinct domains: a targeting domain and a catalytic (toxic) domain. Over time, these toxins have evolved into highly efficient carriers adept at delivering their catalytic domain into cells. The use of biotechnology to manipulate these proteins facilitates the development of potent and exquisitely precise protein ligands designed to specifically target cell surface receptors associated with cancer and deliver treatments in to these cells. Two noteworthy examples of such toxins are the Shiga toxin and the Anthrax toxin.
- targeted therapies
- bacterial AB toxins
- receptors
- high-affinity
- cancer
1. Introduction
Cancer is a worldwide health issue with complex pathophysiology and high mortality rates. Conventional therapies, including chemotherapy, radiation therapy, and surgery, are widely used to eliminate cancer cells and increase the survival rates of patients. However, they encounter several limitations, such as cytotoxicity, multi-drug resistance, and poor selectivity since most therapies cannot distinguish between cancer and normal cells. This lack of targeting results in poor drug accumulation in the tumor and low therapeutic efficacy. Therefore, it is necessary to search for new therapies that can specifically deliver the therapeutic agent to the tumors in a way that reduces adverse effects and significantly improves efficacy [1,2][1][2].
In the search for more accurate therapies, molecular-targeted therapies have emerged as an alternative to conventional therapies as they can deliver the therapeutic agent to the tumors in a way that reduces adverse effects and significantly improves efficacy [1,2][1][2]. They can be used alone or in combination with standard chemotherapy agents [3]. These therapies include the use of small molecules with relatively low molecular weight, monoclonal antibodies, or immunotherapeutic cancer vaccines. They block the growth and spread of tumors through drugs or other substances that target specific molecules in the tumor cells or the tumor microenvironment (cancer-associated fibroblasts, immune cells, cancer stem cells, and vascular endothelial cells) [3,4][3][4]. Typical targets include cell surface antigens, growth factors, receptors, or signal transduction pathways, which, although often mutated or overexpressed in tumors, may also be present in normal tissues [3]. Although these therapies have shown successful results in the treatment of cancer, clinical resistance to these agents is still a major issue. For example, mutations of the monoclonal antibody target and downstream signaling molecules usually lead to acquired resistance due to the activation of alternative growth or survival signaling pathways [4]. Thus, it is essential to search for new ligands and target receptors for improved molecular-targeted cancer therapy.
In this field, some biotoxins have emerged as novel opportunities. Biotoxins are highly efficient natural ligands that arise in almost all forms of life, mainly for defensive purposes against predators [5,6,7][5][6][7]. This term includes a heterogeneous group of extremely poisonous biomolecules (small molecular compounds, proteins, and peptides) produced by the metabolism of numerous living organisms, including bacteria (bacterial toxins), fungi (mycotoxins), plants (phytotoxins), and vertebrate and invertebrate animals (zootoxins) [5,6,8][5][6][8]. These molecules interfere with and disrupt the physiological processes of competing organisms, and, depending on their chemical nature, they exert dose-dependent pathophysiological injury when inhaled, ingested, or absorbed [5,6,9][5][6][9]. One of the most appealing aspects of biotoxins is that they trigger really quick responses due to their high specificity and affinity for their receptors or intracellular molecular targets [10], being lethal at very low doses (LD50 < 25 mg/kg) [11]. Some of these receptors are described as being overexpressed by both, tumor cells and endothelial cells of the neovasculature of solid tumors [11,12,13,14,15,16][11][12][13][14][15][16]. Their inherent specificity and affinity for the recognition of their targets make them an invaluable source of bioactive ligands for use as pharmacological or therapeutic tools for molecularly targeted therapies. Apart from that, biotoxins are also useful tools for studying the physiological role of their molecular targets (e.g., ion channels, receptors, etc.), understanding and treating human diseases, destroying disease vectors, or treating microbial and parasitic infections [6,7,9,14][6][7][9][14]. Depending on the target tissue, biotoxins can be classified as (i) cytotoxins, such as Ricin toxin (Rtx) (Figure 1a); (ii) enterotoxins, such as Staphylococcal enterotoxins (SEs) (Figure 1b); or (iii) neurotoxins, including Batrachotoxin, Botulinum (BoNTs), and Tetanus neurotoxins (TeNT), the most potent toxins known worldwide (Figure 1c) [6,17,18,19,20,21,22,23,24][6][17][18][19][20][21][22][23][24].
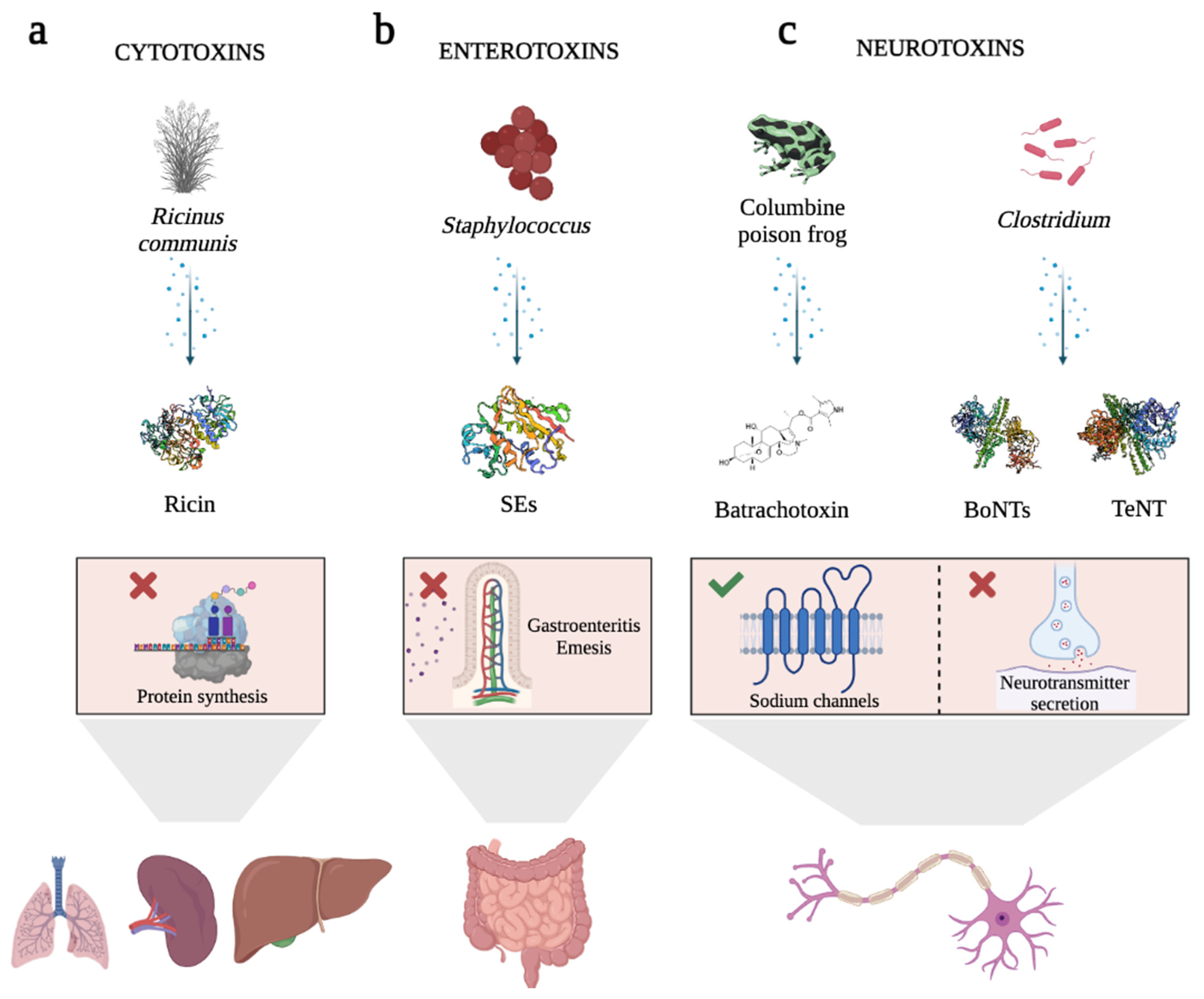
Figure 1. Schematic representation of the cellular effects of highly potent biotoxins. (a) Ricin toxin (Rtx), a protein produced by the seeds of the castor bean plant, has inhibitory effects on protein synthesis. When Rtx is ingested or inhaled, it leads to tissue damage in various organs. (b) Staphylococcal enterotoxins (SEs) are pyrogenic proteins that cause gastroenteritis and emesis. They induce the release of inflammatory molecules by immune system cells. (c) Examples of neurotoxins. Batrachotoxin, a steroid alkaloid found in the skin of the Columbine poison frog, exhibits selectivity in binding to sodium channels. This binding action causes the channels to remain open, leading to heightened permeability to sodium ions. Consequently, it induces cardiotoxicity and neurotoxicity. Among the bacterial toxins, Botulinum neurotoxins (BoNTs) and Tetanus neurotoxin (TeNT) are noteworthy examples. Produced by Clostridium species, these toxins hinder the presynaptic release of the neurotransmitter acetylcholine, thereby resulting in reversible paralysis. BoNTs primarily impede acetylcholine release in peripheral cholinergic terminals, while TeNT targets inhibitory interneurons within the spinal cord. In the graphical representation, inhibition is denoted by a red cross, while activation is indicated by a green tick. Rtx, SEs, BoNTs, and TeNT are represented as cartoon structures using Biorender software. Toxin protein structures Data Bank IDs are 2AAI for Ricin, 1SXT for SEs, 1S0F for BoNTs, and 5N0C for TeNT.
In recent years, significant focus has been directed toward a class of biotoxins known as AB toxins. These toxins, predominantly synthesized by bacteria, have garnered considerable interest. However, it is noteworthy that AB toxins, like the Ricin toxin, can also originate from other sources, including plants. AB toxins are characterized by their unique protein structure, which consists of two distinct domains that serve different functions. Through evolutionary processes, these toxins have evolved into highly efficient carriers capable of transporting the toxic subunit into cells. This transport is facilitated by the binding of the non-toxic ligand subunit to specific cell surface receptors [25]. The molecular recognition and targeted invasion mechanisms employed by AB toxins offer valuable insights for the development of precise therapeutic approaches. The receptor-binding domain, known as the B moiety, plays a crucial role in coordinating binding to host cells and facilitating the delivery of the A moiety into the host cell cytosol. Once inside, the A moiety exerts its enzymatic effects on the cellular machinery [26,27][26][27]. Most of these toxins trigger major human disorders, usually gastrointestinal symptoms, and cause the deaths of millions of people worldwide each year [28]. However, emerging research has revealed that, when appropriately modified, AB toxins exhibit promising characteristics as adjuvants for immune stimulation, autoimmunity suppression [29], and a wide range of biomedical applications [7,14,26,29,30,31][7][14][26][29][30][31]. Furthermore, their potential applications extend to areas such as cancer diagnosis and treatment [32,33][32][33].
2. Generalities about AB Toxins
2.1. Structural Insights of AB Toxins
AB toxins are a class of toxins that contain two distinct components: the active catalytic domain (A) and the receptor binding domain (B) (Figure 2a). The B domain is responsible for coordinating the binding of the toxin to host cells and facilitating the delivery of the A domain into the host cell cytosol. This allows the A domain to exert its enzymatic effect by modifying host proteins and causing cellular changes or intoxication. In most AB toxins, the B domain also includes a translocation domain that plays a crucial role in facilitating the delivery of the A domain into the cytosol. It often forms a pore or channel that allows the A domain to traverse the membrane barrier [26,27][26][27].
Figure 2. Scheme of the active forms of the different types of AB toxins. (a) AB toxins have two moieties: A and B. The A moiety is the active domain with enzymatic activity, while the B domain has the binding receptor property. Depending on the type of AB toxins, they can also have a linker between A and B that usually consists of a peptide and/or a disulfide bond. AB toxins active form results from a proteolytic cleavage between A and B moieties or within the A or B moiety. These toxins can be classified according to their A:B stoichiometry. (b) Single-chain AB toxins are produced as single polypeptide chains. They have a 1:1 A B stoichiometry, and both subunits remain linked by an interchain disulfide bond in the active form. (c) When the B domain is in an oligomeric state, the A and B moieties are produced as separate proteins, which assemble later on. In AB2 and AB5 toxins, A and B are assembled after the synthesis to form the holotoxin. In the active form, proteolytic cleavage occurs within the A domain, giving rise to the A1 and A2 domains that remain linked by a disulfide bond. (d) In A + B toxins, A and B are assembled into the de-active form after the B moiety suffers proteolytic cleavage. In this case, the holotoxin is usually in AB7 form.
2.2. The Structure–Function Relationship of AB
2
-AB
5
Toxins
The AB2 and AB5 toxins consist of two and five identical B subunits, respectively, associated with a single catalytic subunit. The holotoxin is assembled just after the synthesis of both chains. A notable feature of the catalytic A subunit of AB5 toxins is that, although it is a single polypeptide, after proteolytic cleavage, the active form results in two domains (A1 and A2) linked by a disulfide bond. A1 is the catalytic part itself, and A2 is an α-helix that is non-covalently inserted into the central pore that forms when the B-subunit pentamer associates [27,28][27][28] (Figure 2c). Upon reduction of the A domain intrachain disulfide bond, the catalytic domain is delivered into the cytosol [26,40,41,42,43][26][34][35][36][37]. AB5 toxins are subdivided into four different families according to their A subunit sequence homology and their catalytic activity: the (i) Cholera toxin (Ctx) family, (ii) Pertussis toxin family, (iii) Shiga toxin (Stx) family, and the (iv) Subtilase cytotoxin (SubAB) [28].2.3. The Structure-Function Relationship of A + B Toxins
The A + B toxins are produced as two separate polypeptides that do not form the holotoxin complex immediately. In these toxins, the B domain needs to undergo processing by host furin proteases on the cell surface to assemble into the oligomeric form. This moiety then recruits the A moiety to form the holotoxin. The AB7 stoichiometry refers to the presence of seven identically processed B moieties associated with a single A subunit (Figure 2d). This is the case with the Anthrax toxin (Atx) or C2 toxin [44,45,46][38][39][40].2.4. AB Toxins Entry Mechanisms into Cells
AB toxin entry into cell pathways relies on the B subunit’s recognition of receptors displayed on the cell surface, which can be both proteins and glycolipids. Receptor specificity is critical for the pathogenic process because it determines the susceptibility of the host, tissue tropism, and the nature and spectrum of the resultant pathology [26]. The mechanism by which AB toxins reach the cytosol is receptor-mediated endocytosis (RME). They seem to share a common mechanism of action that involves: (i) binding to specific receptors on the surface of cells displaying the receptor; (ii) internalization or translocation across the membrane; and (iii) interaction with the intracellular target [42,43][36][37]. These toxin receptors are typically distributed in lipid rafts enriched in cholesterol and glycosphingolipids [47,48][41][42]. Fluorescence microscopy studies have revealed that certain toxins, upon interaction, lead to the clustering of ligand-receptor complexes in specialized regions of the membrane called coated pits during RME. These coated pits are dependent on the presence of clathrin, a structural protein involved in vesicle formation. Pharmacological experiments have suggested that the clustering step of ligand-receptor complexes in coated pits is mediated by transglutaminase, which facilitates this clustering by generating epsilon-(gamma-glutamyl)-lysine bonds through cross-linking. This process of clustering and concentration of toxin molecules in specific regions of the cell surface is an important mechanism for the internalization of ligand-receptor complexes during endocytosis [42,49,50][36][43][44]. It allows for efficient uptake of the toxins by the cells, ultimately leading to their internalization and subsequent intracellular transport. Some other AB toxins enter via clathrin-independent endocytosis, through cholesterol-rich plasma membrane domains called caveolae [51][45]. But, despite the endocytic carrier, after receptor binding, all toxin–receptor complexes are initially delivered to early endosomal membranes [49[43][45],51], following two different pathways to translocate the A domain into the cytosol. The first pathway involves the release of the A domain from endosomes into the cytosol as a result of polypeptidic conformational changes caused by low endosomal pH. This mechanism is observed in toxins such as Dtx, botulinum toxin, tetanus neurotoxin, Atx, and C2 toxin. In contrast, certain toxins bind to membrane receptors that undergo retrograde transport, leading them to be recycled through the trans-Golgi network. This transport mechanism enables the toxins to reach the lumen of the endoplasmic reticulum (ER) via the Golgi apparatus. Once in the ER, the toxins are released into the cytosol, effectively bypassing the endo-lysosomal pathway. These include single-chain AB toxins, such as exotoxin A, ricin toxin, AB2 toxins, and AB5 toxins [28,41,51][28][35][45]. Based on the structure of AB toxins and the translocation pathway of the A subunit into the cytosol, these modular toxins can be categorized into four distinct groups. Toxins of group 1 include Dtx, BoNTs, and TeNT. They are single-chain AB toxins, where the A domain translocates in the endosome (Figure 3a). After a proteolytic cleavage in the peptide chain, in the active form, A and B remain linked by a disulfide bond. In the endosome membrane, the B domain forms a pore to translocate the A subunit into the cytosol after the reduction of the disulfide bond. Group 2 includes Atx and C2 toxins. They are A + B toxins, where A translocates in the endosome (Figure 3b). The heptamer-shaped ring forms a pore in the membrane of the endosome to translocate A into the cytosol. Toxins of group 3 include Ctx and Stx. They are AB2 or AB5 toxins where the A module translocates in the endoplasmic reticulum (Figure 3c). The proteolytic cleavage occurs in A, resulting in two fragments (A1 and A2) that remain linked by a disulfide bond. In the ER, A1 is translocated to the cytosol with the assistance of ER machinery (Sec proteins), which requires the reduction of the disulfide bond. Finally, group 4 includes Exotoxin A and Ricin toxin. These toxins are also single-chain AB toxins. However, A translocates in the endoplasmic reticulum (Figure 3d) [52][46].
Figure 3. AB toxins classification, according to their structure and A translocation into the cytosol. (a) Toxins of group 1 (Dtx, BoNTs, TeNT) are produced as single-polypeptide precursors. These proteins are proteolytically cleaved to generate a di-chain linked by an inter-chain disulfide bond. In the endosome membrane, the B subunit forms a pore to translocate the A subunit into the cytosol. (b) Toxins of group 2 (Atx, C2 toxin) are produced as separated A and B proteins. The B moiety is activated by proteolytic cleavage by host furin proteases and assembles into heptamer-shaped ring structures that can bind the A moiety. B heptamers form a pore in the membrane of the endosome to translocate the A subunit into the cytosol. (c) Toxins of group 3 (Stx, Ctx), as in group 2, are synthesized as independent A and B proteins. However, proteolytic cleavage occurs in the A moiety, resulting in two fragments that remain linked by a disulfide bond. In the ER, A1 is translocated to the cytosol with the assistance of ER machinery (Sec proteins). This step requires the reduction of the disulfide bond. (d) Finally, toxins of group 4 (Rtx, Exotoxin A) are similar to toxins of group 1, but translocation of the A subunit occurs in the ER instead of the endosomes. Scissors indicate the proteolytic cleavage spot on the polypeptide toxins.
References
- Naz, S.; Wang, M.; Han, Y.; Hu, B.; Teng, L.; Zhou, J.; Zhang, H.; Chen, J. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. Int. J. Nanomed. 2019, 14, 2533.
- Yu, H.; Ning, N.; Meng, X.; Chittasupho, C.; Jiang, L.; Zhao, Y. Sequential Drug Delivery in Targeted Cancer Therapy. Pharmaceutics 2022, 14, 573.
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196.
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34.
- Vijayakumar, A.; Shobha, G.; Moses, V.; Soumya, C. A review on various types of toxins. Pharmacophore 2015, 6, 181.
- Janik, E.; Ceremuga, M.; Bijak, J.S.; Bijak, M. Biological Toxins as the Potential Tools for Bioterrorism. Int. J. Mol. Sci. 2019, 20, 1181.
- Shapira, A.; Benhar, I. Toxin-based therapeutic approaches. Toxins 2010, 2, 2519–2583.
- Pitschmann, V.; Hon, Z. Military importance of natural toxins and their analogs. Molecules 2016, 21, 556.
- Herzig, V.; Cristofori-Armstrong, B.; Israel, M.R.; Nixon, S.A.; Vetter, I.; King, G.F. Animal toxins—Nature’s evolutionary-refined toolkit for basic research and drug discovery. Biochem. Pharmacol. 2020, 181, 114096.
- Zhang, Y. Why do we study animal toxins? Zool. Res. 2015, 36, 183–222.
- Nanda, A.; St. Croix, B. Tumor endothelial markers: New targets for cancer therapy. Curr. Opin. Oncol. 2004, 16, 44–49.
- Cryan, L.M.; Rogers, M.S. Targeting the anthrax receptors, TEM-8 and CMG-2, for anti-angiogenic therapy. Front. Biosci. 2011, 16, 1574–1588.
- Aureli, M.; Mauri, L.; Ciampa, M.G.; Prinetti, A.; Toffano, G.; Secchieri, C.; Sonnino, S. GM1 Ganglioside: Past Studies and Future Potential. Mol. Neurobiol. 2016, 53, 1824–1842.
- Lingwood, C. Therapeutic Uses of Bacterial Subunit Toxins. Toxins 2021, 13, 378.
- Davis-Fleischer, K.M.; Besner, G.E. Structure and function of heparin-binding EGF-like growth factor (HB-EGF). Front. Biosci. 1998, 3.
- Tsujioka, H.; Yotsumoto, F.; Hikita, S.; Ueda, T.; Kuroki, M.; Miyamoto, S. Targeting the heparin-binding epidermal growth factor-like growth factor in ovarian cancer therapy. Curr. Opin. Obstet. Gynecol. 2011, 23, 24–30.
- Lubran, M.M. Bacterial Toxins. Ann. Clin. Lab. Sci. 1988, 18, 58–71.
- Sowa-Rogozińska, N.; Sominka, H.; Nowakowska-Gołacka, J.; Sandvig, K.; Słomi, Ń.; Ska-Wojew, Ó.; Dzka, M. Intracellular Transport and Cytotoxicity of the Protein Toxin Ricin. Toxins 2019, 11, 350.
- Etemad, L.; Moshiri, M.; Hamid, F. Ricin Toxicity: Clinical and Molecular Aspects. Rep. Biochem. Mol. Biol. 2016, 4, 60.
- Etter, D.; Schelin, J.; Schuppler, M.; Johler, S. Staphylococcal Enterotoxin C—An Update on SEC Variants, Their Structure and Properties, and Their Role in Foodborne Intoxications. Toxins 2020, 12, 584.
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front. Microbiol. 2018, 9, 436.
- Cataldi, M. Batrachotoxin. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–9. ISBN 9780080552323.
- Dong, M.; Masuyer, G.; Stenmark, P. Botulinum and tetanus neurotoxins. Annu. Rev. Biochem. 2019, 88, 811–837.
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86.
- Luginbuehl, V.; Meier, N.; Kovar, K.; Rohrer, J. Intracellular drug delivery: Potential usefulness of engineered Shiga toxin subunit B for targeted cancer therapy. Biotechnol. Adv. 2018, 36, 613–623.
- Henkel, J.S.; Baldwin, M.R.; Barbieri, J.T. Toxins from bacteria. Mol. Clin. Environ. Toxicol. 2010, 100, 1–29.
- Zuverink, M.; Barbieri, J.T. Protein Toxins that Utilize Gangliosides as Host Receptors. Prog. Mol. Biol. Transl. Sci. 2018, 156, 325.
- Biernbaum, E.N.; Kudva, I.T. AB5 Enterotoxin-Mediated Pathogenesis: Perspectives Gleaned from Shiga Toxins. Toxins 2022, 14, 62.
- Odumosu, O.; Nicholas, D.; Yano, H.; Langridge, W. AB toxins: A paradigm switch from deadly to desirable. Toxins 2010, 2, 1612–1645.
- Baldauf, K.J.; Royal, J.M.; Hamorsky, K.T.; Matoba, N. Cholera toxin B: One subunit with many pharmaceutical applications. Toxins 2015, 7, 974–996.
- Piot, N.; Gisou van der Goot, F.; Sergeeva, O.A. Harnessing the membrane translocation properties of ab toxins for therapeutic applications. Toxins 2021, 13, 36.
- Bachran, C.; Leppla, S.H. Tumor Targeting and Drug Delivery by Anthrax Toxin. Toxins 2016, 8, 197.
- Robert, A.; Wiels, J. Shiga toxins as antitumor tools. Toxins 2021, 13, 690.
- Eidels, L.; Proia, R.L.; Hart, D.A. Membrane receptors for bacterial toxins. Microbiol. Rev. 1983, 47, 596–620.
- Schmidt, G.; Papatheodorou, P.; Aktories, K. Novel receptors for bacterial protein toxins. Curr. Opin. Microbiol. 2015, 23, 55–61.
- Middlebrook, J.L.; Dorland, R.B. Bacterial toxins: Cellular mechanisms of action. Microbiol. Rev. 1984, 48, 199–221.
- Balfanz, J.; Rautenberg, P.; Ullmann, U. Molecular mechanisms of action of bacterial exotoxins. Zentralblatt Bakteriol. 1996, 284, 170–206.
- Pavlik, B.J.; Hruska, E.J.; Van Cott, K.E.; Blum, P.H. Retargeting the Clostridium botulinum C2 toxin to the neuronal cytosol. Sci. Rep. 2016, 6, 23707.
- Friebe, S.; van der Goot, F.; Bürgi, J. The Ins and Outs of Anthrax Toxin. Toxins 2016, 8, 69.
- Young, J.A.T.; Collier, R.J. Anthrax toxin: Receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 2007, 76, 243–265.
- Abrami, L.; Liu, S.; Cosson, P.; Leppla, S.H.; Van der Goot, F.G. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 2003, 160, 321–328.
- Melton-Celsa, A.R. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiol. Spectr. 2014, 2, 2–4.
- Omaye, S.T. Bacterial toxins. In Food and Nutritional Toxicology; CRC Press: Boca Raton, FL, USA, 2004; pp. 191–214.
- Abrami, L.; Bischofberger, M.; Kunz, B.; Groux, R.; Van Der Goot, F.G. Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors. PLoS Pathog. 2010, 6, 1000792.
- Lord, J.M.; Smith, D.C.; Roberts, L.M. Toxin entry: How bacterial proteins get into mammalian cells. Cell. Microbiol. 1999, 1, 85–91.
- Sun, J.; Sun, J. Roles of cellular redox factors in pathogen and toxin entry in the endocytic pathways. In Molecular Regulation of Endocytosis; IntechOpen: London, UK, 2012.
- Lahiani, A.; Yavin, E.; Lazarovici, P. The Molecular Basis of Toxins’ Interactions with Intracellular Signaling via Discrete Portals. Toxins 2017, 9, 107.
- Schrot, J.; Weng, A.; Melzig, M.F. Ribosome-Inactivating and Related Proteins. Toxins 2015, 7, 1556.
More
