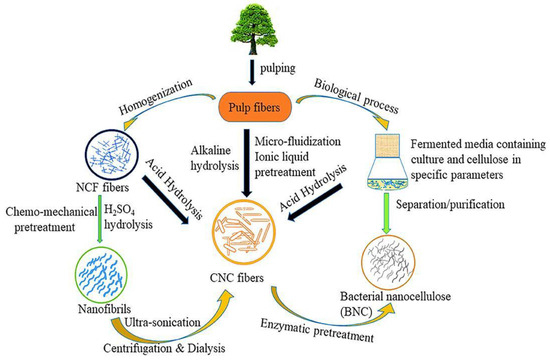
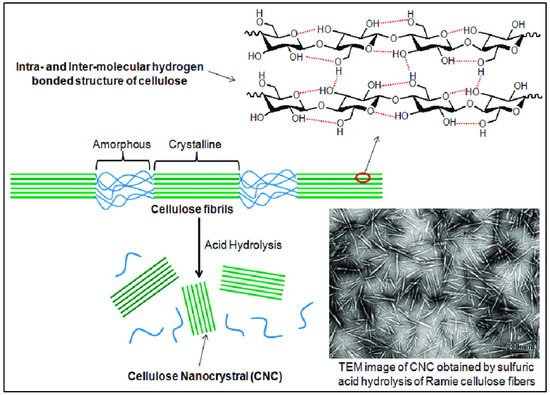
Cellulose is the most abundant natural polymer on Earth. The roles of cellulose nanocrystals’ (CNC) nonlinear geometry in improved performance coatings are appealing for mini structures or device production; yet, CNC is an insulating material. The cellulose nanocrystal (CNC) is a part of the organic crystallization macromolecular compound found in plant fibers and bacteria’s capsular polysaccharides. It has several properties, which include high strength, high crystallization, large physical properties, minimum density, and good biocompatibility. CNCs, with their exceptional mechanical, thermal, and optical properties, have emerged as a versatile and sustainable nanomaterial with the potential to revolutionize various industries.
- cellulose
- cellulose nanocrystals
- mechanical process
- homogenization
- micro fluidization
- fine grinding
- alkali
- acid
- hydrolysis
1. Introduction
2. Mechanical Process of Preparation of CNC
The mechanical process (Figure 2) includes four steps and is a physical approach to obtaining the CNC: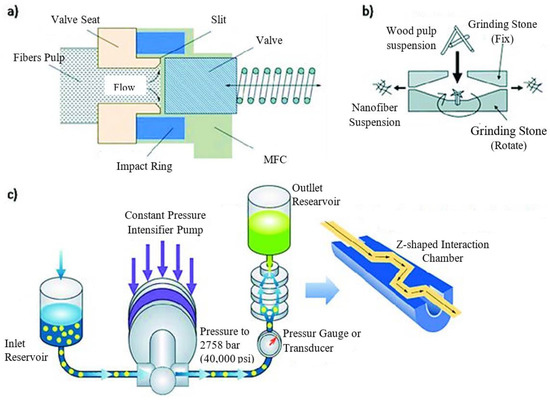
- (i)
-
Homogenization under high pressure;
- (ii)
-
Micro fluidization;
- (iii)
-
Fine grinding;
- (iv)
-
Freezing smashing.
2.1. Homogenization under High Pressure
It takes just one step to homogenize a solid into ultrafine particles in a solution, which results in a stable suspension. High-pressure homogenization was the most common method for producing microcrystalline cellulose (MCC). Several investigations have shown that it can be used to make MCC from various basic materials [14][12].2.2. Micro Fluidization
Figure 3 depicts the technical process of micro fluidization under high pressure, one way of creating nanomaterial. The fluid pump initially injected and compressed the raw slurry to a pressure of roughly 4000 bars. After that, it was thrown into a Y-shaped interactive tank, where two liquid squirts traveling at 1000 m/s collided. The vacuum effect and enormous shear force generated by this impact lead to the production of nanoparticles [15][13].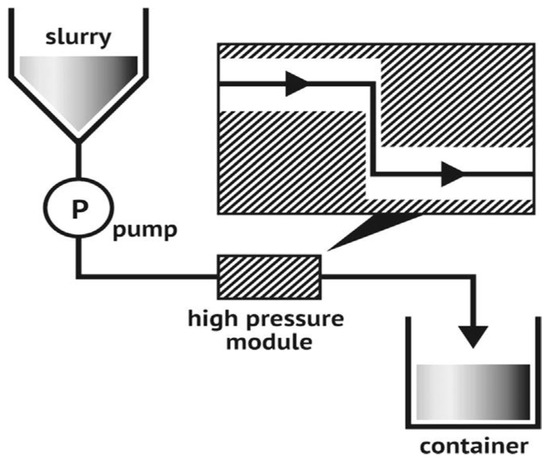
2.3. Fine Grinding
The fine grinder and a commercial grinder worked together to provide a new physical approach to making MCC. It was clear that the main components were made up of two discs, one internal and one exterior, each covered with grooves with a unique set of characteristics. As the two discs rotated in tandem, they applied crushing, shearing, friction, grinding, and ripping forces that caused the fibers to split and shrink. However, it was very inefficient, and only a few instances of its use were documented [15][13]. In Figure 4, we can see the experimental setup for fine grinding can be seen. The raw material is poured into the funnel, and, inside the chamber, the cellulose pulp passes through grilling stones and an adjustable gap. Finally, the finished product is collected from the collection bucket.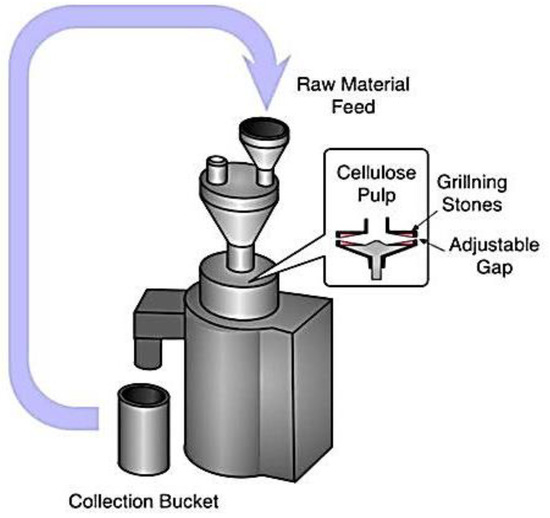
3. Chemical Process
The CNC can only be obtained by partly disrupting glycosidic bonds; chemical hydrolysis makes a strong argument for attaining this. A kind of polysaccharide, cellulose, is created from glucose molecules joined together by the β-1,4-glucosidic link [17][15].3.1. Alkali Hydrolysis
Natural cellulose’s crystal shape changed from I to II after being subjected to a 9-weight-percent NaOH treatment. The alkali has the power to both expand the cellulose and rupture its internal hydrogen bonds. Even though alkali has been used to hydrolyze cellulose in several studies, alkali was mainly used to dissolve lignin and pectin [18][16].3.2. Acid Hydrolysis
Repeating the above structures yielded the cellulose fiber, composed of discontinuous sections of crystalline and amorphous cellulose. The CNC was made from reed pulp utilizing sodium m-nitrobenzene sulfonate (SMS) as a cocatalyst and 55-weight-percent sulfuric acid. The final CNC had an I-form and a rod-like shape, measuring 12 nm in diameter and 146 nm in length. Its crystallinity was 76.1 percent. On the other hand, the CNC-produced thermal insulation foam demonstrated its flawless thermal insulation ability under typical conditions. The ideal time range for the hydrolysis of this fiber was discovered to be between 45 and 55 min [19][17]. We can see the internal structure of cellulose can be seen (Figure 5), where two types of regions are present. An unorganized blue portion is called the amorphous region, and the green liner pattern is called a crystalline region. To obtain CNC from the amorphous cellulose region, concentrated 98% sulfuric acid must be broken.
References
- Koshani, R.; Madadlou, A. A viewpoint on the gastrointestinal fate of cellulose nanocrystals. Trends Food Sci. Technol. 2018, 71, 268–273.
- Kafy, A.; Akther, A.; Shishir, I.; Kim, H.C.; Yun, Y.; Kim, J. Cellulose nanocrystal/graphene oxide composite film as humidity sensor. Sens. Actuators A Phys. 2016, 247, 221–226.
- Jafary, R.; Khajeh Mehrizi, M.; Hekmatimoghaddam, S.H.; Jebali, A. Antibacterial property of cellulose fabric finished by allicin-conjugated nanocellulose. J. Text. Inst. 2015, 106, 683–689.
- Trache, D.; Thakur, V.K.; Boukherroub, R. Cellulose Nanocrystals/Graphene Hybrids—A Promising New Class of Materials for Advanced Applications. Nanomaterials 2020, 10, 1523.
- Kang, X.; Kuga, S.; Wang, C.; Zhao, Y.; Wu, M.; Huang, Y. Green Preparation of Cellulose Nanocrystal and Its Application. ACS Sustain. Chem. Eng. 2018, 6, 2954–2960.
- Tritt-Goc, J.; Lindner, Ł.; Bielejewski, M.; Markiewicz, E.; Pankiewicz, R. Synthesis, thermal properties, conductivity and lifetime of proton conductors based on nanocrystalline cellulose surface-functionalized with triazole and imidazole. Int. J. Hydrogen Energy 2020, 45, 13365–13375.
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240.
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228.
- Matteini, P.; De Angelis, M.; Ulivi, L.; Centi, S.; Pini, R. Concave gold nanocube assemblies as nanotraps for surface-enhanced Raman scattering-based detection of proteins. Nanoscale 2015, 7, 3474–3480.
- Gupta, G.K.; Shukla, P. Lignocellulosic Biomass for the Synthesis of Nanocellulose and Its Eco-Friendly Advanced Applications. Front. Chem. 2020, 8, 601256.
- Yang, Y.; Chen, Z.; Zhang, J.; Wang, G.; Zhang, R.; Suo, D. Preparation and Applications of the Cellulose Nanocrystal. Int. J. Polym. Sci. 2019, 2019, 1767028.
- Vilela, C.; Pinto, R.J.B.; Figueiredo, A.R.P.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. 1 Development and applications of cellulose nanofibres based polymer nanocomposites. In Advanced Composite Materials: Properties and Applications; De Gruyter Open: Berlin, Germany, 2017; pp. 1–65. ISBN 978-3-11-057443-2.
- Pyrgiotakis, G.; Luu, W.; Zhang, Z.; Vaze, N.; DeLoid, G.; Rubio, L.; Graham, W.A.C.; Bell, D.C.; Bousfield, D.; Demokritou, P. Development of high throughput, high precision synthesis platforms and characterization methodologies for toxicological studies of nanocellulose. Cellulose 2018, 25, 2303–2319.
- Kim, J.-M.; Kim, H.-N.; Park, Y.-J.; Ko, J.-W.; Lee, J.-W.; Kim, H.-D. Fabrication of transparent MgAl2O4 spinel through homogenous green compaction by microfluidization and slip casting. Ceram. Int. 2015, 41, 13354–13360.
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.A.; Ikkala, O. Hydrophobic Nanocellulose Aerogels as Floating, Sustainable, Reusable, and Recyclable Oil Absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816.
- Mousa, M.H.; Dong, Y.; Davies, I.J. Recent advances in bionanocomposites: Preparation, properties, and applications. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 225–254.
- Chakrabarty, A.; Teramoto, Y. Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them. Polymers 2018, 10, 517.
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002.