Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Mohammad Alnajideen.
This aentrticley reviews the critical role of material selection and design in ensuring efficient performance and safe operation of gas turbine engines fuelled by ammonia–hydrogen. As these energy fuels present unique combustion characteristics in turbine combustors, the identification of suitable materials becomes imperative. Detailed material characterisation is indispensable for discerning defects and degradation routes in turbine components, thereby illuminating avenues for improvement. With elevated turbine inlet temperatures, there is an augmented susceptibility to thermal degradation and mechanical shortcomings, especially in the high-pressure turbine blade—a critical life-determining component. This review highlights challenges in turbine design for ammonia–hydrogen fuels, addressing concerns like ammonia corrosion, hydrogen embrittlement, and stress corrosion cracking. To ensure engine safety and efficacy, this article advocates for leveraging advanced analytical techniques in both material development and risk evaluation, emphasising the interplay among technological progress, equipment specifications, operational criteria, and analysis methods.
- gas turbine
- materials characterisation
- ammonia
- hydrogen
- blades
1. Introduction
As we move towards a more sustainable and net-zero emission era, innovative power generation systems that leverage alternative energy fuels are gaining significant interest worldwide. The potential of hydrogen (H2) as an energy source to support a low-carbon economy has attracted considerable attention in ongoing research, reflecting the increasing urgency to mitigate the adverse impacts of climate change and global warming. However, numerous technical challenges exist, particularly concerning the storage, distribution, and sustainable usage of hydrogen [1]. Consequently, a growing body of scientific work is currently investigating the feasibility of indirect storage methods [2]. In particular, chemicals such as ammonia (NH3) are being examined as viable alternatives for hydrogen carriers and storage. Interestingly, ammonia shows promise as a potential alternative energy carrier for hydrogen due to its high hydrogen energy density, low storage costs per unit volume, and the ease of its storage and transportation processes, thus presenting it as a feasible option for energy fuels [2]. It is worth noting that ammonia is widely used as a fertiliser in agro-industries, implying that there is an already well-established infrastructure for its production, storage, and distribution. Despite those advantages, there are extant limitations and research gaps that need to be addressed. A major challenges is that ammonia exhibits unique combustion characteristics compared with hydrocarbon fuels. It has a relatively low burning velocity (~7 cm/s), low flame temperature, narrow flammability range, and requires a higher ignition energy [3,4][3][4]. It is important to note that the combustion characteristics may vary based on the conditions under which combustion occurs, such as but not limited to, temperature, pressure, and the fuel–air mixture.
Combustion of ammonia can lead to high levels of nitrogen oxide (NOx) emissions, contributing to air pollution [5]. These challenges, however, are not insurmountable, and potential solutions may involve fuel blending, incorporating fuels such as hydrogen or methane with ammonia. By doping ammonia with these fuels, the burning velocity can be improved, and the reaction can be more effectively regulated [6,7][6][7]. Such blends also bear the potential to reduce NOx emissions, thereby increasing the feasibility of ammonia as an energy production medium [6,8,9][6][8][9]. Further investigation is required to determine the viability of ammonia-fuel blends as a low or zero-carbon energy source and to understand any potential environmental consequences. Renowned researchers in the field, including Valera-Medina et al., 2018 [2] and Kobayashi et al., 2019 [10], have demonstrated that to fully harness the potential of ammonia as an energy source, a thorough understanding of the combustion dynamics and associated chemical reactions is essential. This comprehensive understanding would provide a foundation for addressing and overcoming the challenges presented by ammonia’s inherently low flammability and the issue of NOx emissions during combustion.
2. Characteristics of Ammonia–Hydrogen Combustion in Gas Turbines
Understanding how ammonia–hydrogen reacts with materials is essential. Thus, studying their relevant characteristics in gas turbines becomes important.2.1. Hydrogen in Gas Turbines
Since its invention in the early 20th century, the gas turbine, normally fuelled by natural gas and diesel, has been used for electricity generation, propulsion and aviation [23][11]. Hydrogen can be combusted in a gas turbine [24][12]; however, the distinction between hydrogen and other hydrocarbon fuels introduces complexities when transitioning to hydrogen usage. Given that turbines have been principally designed and optimised for natural gas combustion, these variances necessitate specific modifications to the turbines to facilitate a complete hydrogen combustion [25,26][13][14]. The challenges associated with hydrogen combustion mainly arise from its high reactivity and unique physical properties [25][13]. Hydrogen’s high reactivity causes higher flame speed and flame temperatures, and lower auto-ignition delay [27,28][15][16]. High flame speeds can result in combustion occurring outside the designated combustion zone. High flame temperatures can lead to significant challenges including the degradation of turbine materials and the need for advanced cooling techniques [29,30][17][18]. Furthermore, the elevated temperatures can compromise turbine efficiency, introduce combustion instabilities, and alter acoustic signatures, potentially causing resonance phenomena [31,32][19][20]. Moreover, the distinctive flame characteristics of hydrogen need vigilant operational management, given its broad flammability limits, and raise concerns regarding both erosion of turbine components and the overarching safety [33,34][21][22]. The lower autoignition temperature for hydrogen denotes a reduced energy need for initiating combustion, which potentially can cause the combustion reaction to occur too early [35][23]. Hydrogen’s flame speed significantly exceeds that of natural gas by over threefold, implying conventional combustors may not be able to handle hydrogen’s flame dynamics, thus risking damage [32,35][20][23]. Given hydrogen’s lower volumetric heating value, triple the flow rate is required for equivalent power outputs compared with natural gas. Thus, gas turbines that utilise hydrogen demand flow rate adjustments [32][20]. In addition, hydrogen’s minuscule molecular structure might induce leakages through components designed for natural gas, presenting risks due to its invisibility and flammability. Transitioning to hydrogen offers the benefit of CO2 emissions reduction, although its combustion might influence NOx emissions [24,28,32,35][12][16][20][23]. To overcome these issues, one might consider the deployment of advanced combustion techniques, utilising improved turbine materials and enhancing operational controls. Adjusting the turbine’s compressor may address the lower volumetric heating value of hydrogen. Implementing redesigned piping, enclosures, and flame detectors might mitigate associated hazards [25,36][13][24]. Nevertheless, the most fundamental alteration pertains to the combustor [37][25]. Thermodynamically, temperature and pressure are critical variables that affect the cycle’s effectiveness and characteristics. Gas turbines with a higher inlet pressure result in improved exergy and thermal efficiency, while those with a higher outlet pressure decrease these attributes. The cycle’s exergy and thermal efficiency are directly affected by higher turbine inlet temperatures [38][26]. The flame temperature generally increases with the hydrogen fraction in fuel mixtures. The curve of the flame temperature exhibits two linear regimes, with a higher rate of temperature growth when the hydrogen (H2) fraction exceeds 90%, indicating H2 dominance in the second regime, as shown in Figure 31 [39,40][27][28]. Hydrogen-doped ammonia can be used as a carbon-free fuel, and NH3/H2/air flames attain higher maximum flame temperatures with higher H2 content [41][29]. All equivalence ratios studied have shown increased normalised heat release rates with H2 addition. With an increase in hydrogen proportion, maximum heat release occurs at lower temperatures in stoichiometric and fuel lean zones. Fuel-rich and stoichiometric regions still have a higher rate of heat release in general [39,42][27][30].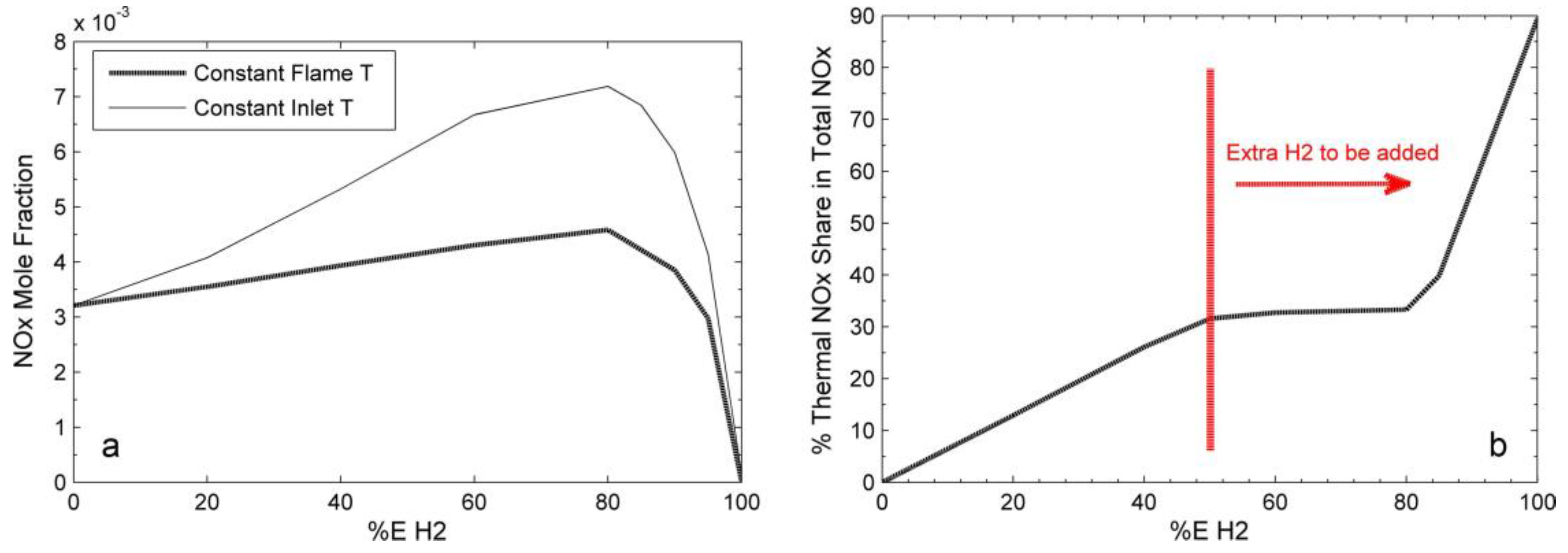
2.2. Ammonia in Gas Turbines
Ammonia has been considered a potential fuel for many decades, with interests peaking at different times, particularly during the energy crises of the 1970s and shifts in energy paradigms [62][50]. The direct utilisation of ammonia as a fuel for internal and external combustion systems is not a new idea or commercial on a large scale. There have been instances of ammonia being used in internal combustion engines between the 1970s and 1980s and other applications on laboratory scales [63][51]. However, ammonia’s potential is due to its widespread production and its storage and handling infrastructure, primarily for agricultural purposes. In terms of global production, ammonia ranks second after sulfuric acid, boasting an annual yield of over 250 million tonnes in 2023 [64][52], making ammonia an indispensable element in agricultural, industrial, and potential clean energy applications worldwide. The interest in ammonia as a fuel in recent years is due to its potential as a carbon-free source, especially when it is synthesised using renewables such as solar and wind power [2]. Its utility as a hydrogen carrier becomes of great interest when it is decomposed. Ammonia serves both as a direct fuel and a hydrogen carrier [2]. Its higher volumetric energy density compared with hydrogen makes it a more spatially efficient storage medium. Despite hydrogen’s superior gravimetric energy density of 120 MJ/kg, its volumetric energy density lags behind conventional fossil fuels with values of 8.49 MJ/L (liquid form) and 4.5 MJ/L (compressed) [65][53]. In contrast, ammonia’s gravimetric and volumetric energy densities are 18.8 MJ/kg and 12.7 MJ/L, respectively [2,65][2][53]. Ammonia storage conditions are more moderate than those for hydrogen, allowing it to be stored as a liquid at ambient temperature with about 10 bar pressure or at −33.4 °C (−28.12 °F) under atmospheric pressure [65][53]. This facilitates more energy-efficient storage with minimal boil-off losses. Its properties, including a high autoignition temperature of 650 °C (1202 °F) and greater density in both liquid and gaseous states compared with hydrogen, make its storage and transport logistics more favourable [66][54]. One drawback, however, is the energy-intensive reconversion of ammonia to hydrogen gas [67][55]. While ammonia’s toxicity warrants caution, existing infrastructure manages this risk effectively [68][56]. In other words, ammonia can overcome the complexities associated with hydrogen storage and distribution, and its complexities are significantly less than those associated with hydrogen. The concept of using ammonia as a fuel has roots going back to the early 20th century, but the exact “first” use can be challenging to pin down due to miscellaneous applications and research initiatives over the years. Ammonia’s potential as an energy carrier is not entirely new, even if its adoption on a large scale is still emerging. During the Second World War, conventional fuel shortage led Belgium to power buses using ammonia [69][57]. A more avant-garde application came in the 1960s when the U.S.A. utilised ammonia as a fuel in the groundbreaking X15 aircraft [70][58]. This period of innovation continued with explorations into ammonia’s viability for gas turbines and reciprocating engines [62][50]. Despite the unidentified outcomes from these studies, the historical precedents set the stage for renewed interest in ammonia as a sustainable energy solution in today’s context. Previous work has shown that the combustion of ammonia presents poor characteristics, including poor reactivity, slow-burning velocity, narrow flammability range, high auto-ignition temperature, and a propensity for excessive NOx emissions [10,71][10][59]. Upon ignition, ammonia exhibited an adiabatic flame temperature lower than both hydrogen and natural gas, being recorded at 1800 °C (3272 °F), 2110 °C (3812 °F), and 1950 °C (3542 °F), respectively. The combined effects of this lower temperature and the absence of CO2 in the resultant gases led to reduced radiative heat transfer, thereby impeding combustion [65,72][53][60]. Ammonia also exhibited lower laminar burning velocities compared with hydrogen and natural gas, with values of 0.07 m/s, 2.91 m/s, and 0.37 m/s, respectively [73,74][61][62]. Furthermore, its narrow flammability range further exacerbated ignition challenges. A significant concern with ammonia combustion is the potential for NOx emissions. Stoichiometric combustion of ammonia did not produce NOx. Nevertheless, under actual conditions, nitrogen-containing radicals might be generated, leading to NOx formation [74][62]. However, NOx removal technologies were mature, and strategies like selective catalytic reduction (SCR) were available to mitigate such emissions [75][63]. Interestingly, ammonia from the fuel might have been utilised for this purpose [65][53]. The presence of potential unburned ammonia presented an issue due to its toxic nature [73][61]. Furthermore, it was found that ammonia could lead to corrosion in materials, warranting careful consideration. Various strategies exist for enhancing the combustion process [76][64]. Using gaseous ammonia over its liquid form, integrating combustion additives, and incorporating a swirler and flame holder are all established methods that have been proven to enhance combustion stability and efficiency while reducing NOx emissions [77,78][65][66]. Another opportunity being explored involves blending with other fuels [79,80][67][68]. This secondary fuel might serve solely as an ignition aid or be part of a continuous fuel blend. Studies have indicated that the efficiency of ammonia combustion can be significantly improved with the inclusion of auxiliary fuels, such as hydrogen and methane [81,82,83][69][70][71]. Combustors, simulating those found in gas turbines, have been tested with a blend of ammonia and hydrogen [84,85,86][72][73][74]. These experimental trials resulted in a significant increase in laminar flame velocity. Nevertheless, this enhancement was accompanied by an increased radical formation, which subsequently escalated NOx emissions and shortened the combustion process’s operational range [65][53]. Conversely, some research indicates that the incorporation of hydrogen can attenuate the NOx formation [2]. Mixtures of ammonia with hydrocarbons have demonstrated augmented flame velocity and enhanced radiation heat transfer [87][75]. A remarkable reduction in CO2 emissions was attributed to a reduced hydrocarbon utilisation, indicating such blends could be key in transitioning from carbon-intensive fuels. However, an increased ammonia concentration might pose risks of heightened NOx emissions [65][53]. The key challenges in combusting ammonia in gas turbines revolve around its limited flammability, combustion instability, and potential NOx emissions [88][76]. Previous research in this domain has largely been confined to small-scale experiments under specific conditions. In Japan, combustion studies involving both pure ammonia and an ammonia–methane blend were combusted in a 50 kW turbine [74][62]. The process’s combustion efficiency was determined by comparing the thermal efficiency (based on the fuel’s LHV) to the efficiency of pure natural gas combustion. Results for pure ammonia floated between 89% and 96%, while the blend showed between 93% and 100%. Furthermore, the turbine exhibited operational flexibility with the mixed fuel, functioning even below 40% of its rated capacity [74][62]. Ammonia was also introduced into a commercial coal-fired power plant in Japan. Although the addition was minimal on an energy scale (0.6–0.8%), there was a notable reduction in CO2 emissions without compromising efficiency [2]. Experimental setups using small-scale gas turbine burners have successfully achieved stable flames with blends containing up to 80%vol ammonia with methane and 50%vol ammonia with hydrogen [89][77]. Ammonia cracking is a process that decomposes ammonia into hydrogen and nitrogen gases, offering a potential source of hydrogen fuel or hydrogen–ammonia blends for gas turbines [74][62]. This process essentially mirrors the synthesis of ammonia [90][78]. The catalyst type determines the necessary operational temperature. For instance, ruthenium-based catalysts are effective at approximately 500 °C, but efforts are underway to identify catalysts that operate at reduced temperatures [62][50]. Regarding capacity, ammonia crackers have been engineered to handle up to 10 tons per hour [91][79]. The debate between direct ammonia utilisation versus its decomposition remains unresolved. While Aziz et al. [65][53] encourage the direct use of ammonia in combustion or fuel cells, Ikäheimo et al. [92][80] support the decomposition of ammonia followed by hydrogen combustion. Alboshmina [16][81] conducted a comprehensive study that introduces the development and evaluation of an innovative cracker system that utilises energy derived from the combustion process for the pre-cracking of ammonia. A distinct geometry, which was validated with testing, has been shown to supply the requisite energy for the cracking mechanism while simultaneously establishing recirculation domains that bolster flame stabilisation. Furthermore, the research succeeded in reducing NOx emission levels by incorporating a minor proportion of the fuel mixture into the area preceding the cracker and following the burner. Both computational and empirical findings ascertain that a specific design—namely, a hemispherically tipped bluff body—positioned at the heart of a swirl combustor can amplify flame resilience (manifested as enhanced resistance to blowoff), cultivate expansive recirculation areas for protracted residence durations, and utilise the anchoring apparatuses that secure the cracker to distribute uncombusted ammonia for NOx regulation objectives. Consequently, the system outlined therein is judged to be suitable for ammonia-based fuel applications, predicting a reduction in NOx emissions while facilitating the effective combustion of ammonia-rich mixtures.2.3. Advancements in Ammonia–Hydrogen Co-Firing in Gas Turbines
In 1936, J. Breton [93][82] measured the detonation velocity of ammonia–oxygen mixture and the results revealed that there are detonation boundaries (25.4–75.0% at 1500 m/s), similar to flammability limits, beyond which consistent detonation is not observed. Information on the phenomenon was experimentally observed. In 1967, Verkampf et al. [94][83] conducted experimental investigations to assess the minimum ignition energy, quenching distance, flame stability limits, and performance in gas turbine burners when utilising ammonia–air mixtures. Relative to propane, which has a minimum ignition energy of less than 0.5 millijoules, ammonia exhibited a significantly higher value of 8 millijoules. Under stoichiometric conditions, the quenching distance of ammonia–air stood at 0.275 inches, in contrast with the recorded 0.08 inches for propane–air. Flame stability assessments showed that ammonia combusted at merely half the air-flow velocity achievable with hydrocarbon fuels, and the stable flame’s equivalence ratio range was notably more restricted than that of hydrocarbon fuels. These results were corroborated during gas turbine burner evaluations. The study concluded that, in the absence of augmentations like enhancing ignition system energy, expanding the combustion liner diameter approximately twofold, and gaseous state ammonia injection, conventional gas turbine burners cannot use pure ammonia as a direct replacement for hydrocarbons. Two strategies to enhance ammonia’s combustion characteristics were explored: the introduction of additives and ammonia’s partial pre-dissociation. Additives, assessed within the flame stability apparatus, constituted 5% by volume of the overall fuel. At this concentration, no additive sufficiently enhanced the flame stability parameters. Notably, 28% dissociated ammonia represented the minimum ignition energy, quenching distance, and flame stability attributes of methane. Subsequent testing of partially dissociated ammonia in a gas turbine burner suggested that gas turbine combustion systems, designed optimally for hydrocarbon fuels, could feasibly utilise 28% dissociated ammonia as an alternative fuel source. Over the years, significant strides have been made in understanding how to effectively use ammonia as an alternative fuel. Many studies have been focused on transportation applications [5], exploring ammonia combustion in spark-ignition (SI) gasoline engines [95,96][84][85] and compression-ignition (CI) diesel engines [97,98][86][87]. In these studies, ammonia is mixed with other fuels such as hydrogen, diesel, gasoline, biodiesel, and other fuels to enhance combustion performance. The results have shown that using ammonia-based fuels for power generation is an enticing prospect, particularly for complementing intermittent renewable energy sources like wind and solar. Given the widespread deployment of gas turbine power plants for electricity generation in recent years and the urgent need to reduce carbon dioxide emissions, the concept of utilising ammonia–hydrogen in gas turbines presents a noteworthy opportunity [9]. Initial research conducted in this century on ammonia combustion in gas turbines illustrates some challenges, such as ammonia’s lower reactivity and the requirement for higher ignition energy compared with conventional fossil fuels [9,99][9][88]. A team of researchers from Cardiff University conducted many studies on the combustion of alternative fuel blends of ammonia/hydrogen/methane in a laboratory-scale generic swirl burner for large combustion applications [89,100,101][77][89][90]. The results from the swirl burner demonstrated the need for an innovative injection approach to maintain combustion stability, particularly when injecting hydrogen with ammonia. Xiao et al. [9] developed a comprehensive chemical–kinetic mechanism to facilitate the use of ammonia–hydrogen blends as a viable alternative fuel source for gas turbine power generation systems. Their mechanism/model renovated and advanced the kinetic mechanism, utilising Mathieu’s model [99][88] as a base. Mathieu’s model was originally built for shock-tube experiments on ammonia ignition delay time measurements under high pressure of up to 30 bars. Xiao’s model accuracy was simulated and examined by evaluating parameters such as NOx emissions, ignition delay times, and laminar burning velocity, with particular attention paid to high-stress conditions usually associated with gas turbine operation. Under such conditions, the model was proficient at predicting various phenomena, including autoignition, flashback, and emission characteristics. Furthermore, in comparison with other mechanisms presented in the study by Xiao et al. [9], the findings exhibited satisfactory accuracy of the proposed model under varying practical equivalence ratio conditions. In a numerical study conducted by Hewlett et al. [72][60], the feasibility of utilising by-product ammonia, generated from every tonne of steel produced using blast furnace processes within the steelworks industry, for gas turbine power generation was investigated. This ammonia, largely obtained in a vapour state, is created from the purification process of coke oven gas (COG). In 2017, the global production of by-product ammonia within the steel industry was estimated at 1.7 Mt. This inspires researchers to investigate by-product ammonia present in the waste streams of many other industries such as oil refining, dairy farming, and biomass processing. CHEMKIN-PRO software was utilised to determine the optimal proportion of ammonia vapour, and in a separate instance, anhydrous ammonia derived from said vapour, when mixed with COG or methane at equivalence ratios ranging between 1.0 and 1.4 under an elevated input temperature of 550 K. Under this condition for pure anhydrous ammonia, NOx and CO concentrations were found to range from 600 ppm to > 20 ppm and 0 ppm to 10,000 ppm, respectively, when the equivalence ratio varied from 0.75 to 1.40. A Brayton–Rankine cycle, incorporating integrated recuperation, was designed using Aspen Plus software. Efficiencies for the entire cycle were calculated based on a set of favourable equivalence ratios, as determined from combustion simulations. These findings subsequently reported a sequence of emissions testing in a representative gas turbine combustor. The study predicted that 15%vol addition of steelwork COG, at an inlet temperature of 550 K, may contribute to the reactivity of ammonia-blended fuels, whilst reducing undesirable emissions. A detailed experimental investigation was carried out by Hewlett et al. [71][59] following their numerical analyses in Ref. [10] using a premixed swirl burner in a model GT combustor, previously used in the successful combustion of NH3/hydrogen blends, with favourable NOx and unburned fuel emissions. The study focused on ammonia in the industrial wastewater of steelworks. This by-product ammonia was present in an aqueous blend of 60–70%vol water and was normally destroyed. Continuing their research, the addition of 10, 15, and 20%vol COG to each NH3-based fuel was investigated experimentally at 25 kW power with inlet temperatures >500 K, at atmospheric pressure. The study also investigated the combustion performance of combining anhydrous and aqueous by-product NH3 in an approximate 50:50%vol blend, comparing the performance with that of each unblended ammonia source. The results confirmed their predicted numerical analyses. NO levels of <200 ppm and <300 ppm from the combustion of 15% COG with both ammonia and a 70% ammonia, 30% water blend were achievable. However, further work is required to find the optimum equivalence ratio for the blends and to predict the ideal fuel-rich primary zone operating conditions. A number of related experimental studies undertaken by Cardiff University’s Gas Turbine Research Centre (GTRC) and Centre of Excellence on Ammonia Technologies demonstrated the potential to use lean premixed NH3/H2 mixtures in a staged model GT combustor, with NOx and unburned fuel concentrations <50 ppm [104][91]. Mao et al. [105][92] investigated the effects of equivalence ratio, inlet temperature, and pressure on NO demission and primary laminar burning velocity for two-stage combustion of 70%NH3/30%H2 (by vol). Figure 52 illustrates the chemical reactor network model that was developed using CHEMKIN-PRO software for a gas turbine burner. The variations in NO mole fraction, as observed with changes in both the primary and total equivalence ratios, highlighted the significant role of the primary equivalence ratio in NO emissions at atmospheric pressure. A primary equivalence ratio of 1.25 was found as the most effective ratio for minimising NO emissions. As pressure increased, NO concentrations at different primary equivalence ratios exhibited distinct trends with pressure. The pressure effect was split into two parts: (1) it suppresses NO formation in the primary combustion zone by reducing the thickness of the primary flame and (2) it enhances thermal NO formation in the lean combustion zone. Hence, considering the combined impact of pressure in these two combustion zones, the NO emission could be maintained below 100 ppm at a total equivalence ratio of 0.6, provided the pressure exceeds 0.5 MPa, regardless of the primary equivalence ratio. An examination of the influence of inlet temperature and pressure on laminar burning velocity revealed that the increase in the laminar burning velocity with the increase in inlet temperature was more significant than the reduction effect of pressure. Moreover, elevated pressure significantly reduced NO emissions, enabling the achievement of low NO concentrations, even at high inlet temperatures, when pressurised. Consequently, conditions of high inlet temperature and pressurisation are viable for enhancing both flame propagation and NO emission control of the NH3/H2 fuel mixture. At pressurised conditions, it is feasible to achieve NO emissions of less than 200 ppm and a laminar burning velocity exceeding 0.2 m/s, for inlet temperatures ranging between 500 K and 600 K.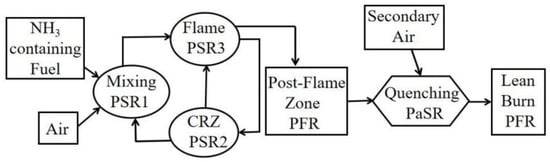
Since 2014, Cardiff University’s research centres, including the Gas Turbine Research Centre (GTRC), Net-Zero Innovation Institute (NZII), and Centre of Excellence on Ammonia Technologies (CEAT), have been actively involved in investigating the use of ammonia–hydrogen and ammonia–methane blends. The successful completion of several collaborative projects involving industrial and academic organisations from across the globe has been a result of their research endeavours [11][131]. Blends of ammonia and hydrogen were evaluated by gradually increasing the ammonia concentration in increments of 10% (vol %) NH3, starting from 50% NH3 (vol %) with the remaining gas comprising hydrogen [148][132]. The study results revealed that the optimal blend, which exhibited the lowest unburned ammonia content and the highest flame temperature, was the 60–40% NH3-H2 mixture. However, this blend also demonstrated high levels of NO emissions. To address this issue, a small amount of NH3/H2 mixture (X = 4%) was injected downstream of the primary zone in a newly designed burner that promoted circulation, thereby enhancing the residence time and reducing the NO emissions in the exhaust gas. The findings of further investigation pertain to the unburned NH3 and NOx emissions, namely, NO, NO2, and N2O, under diverse operational conditions when using NH3-H2 blends in a tangential swirl burner that simulates industrial gas turbines [149][133]. The outcomes suggest that NOx emissions can be effectively balanced at equivalence ratios close to 1.05–1.2, while also providing a deeper understanding of the chemical mechanisms that govern the generation and elimination of these undesirable emissions. This knowledge is expected to contribute towards the wider implementation of NH3-based energy systems.
3. Challenges in Design and Material Selection for Gas Turbines
3.1. Existing Gas Turbine Technologies
A gas turbine is a class of heat engines that convert the chemical energy stored in fuel into mechanical energy and is extensively used in power generation, marine, propulsion, and aircraft. The fundamental working principle of a gas turbine involves the combustion of fuel with compressed air, which leads to the production of high-temperature high-pressure gas. This gas, flue gas, is then made to expand through a turbine, thereby driving a generator or other machinery [157][141]. Although the fundamental principle of gas turbines used in aircraft, commonly referred to as “jet engines”, is similar to that of conventional gas turbines, the end result is distinct, as the jet engines generate the required thrust for the aircraft to move forward [15][142]. The distinction between the gas turbine used in aircraft and its stationary counterpart used in power stations is illustrated in Figure 74.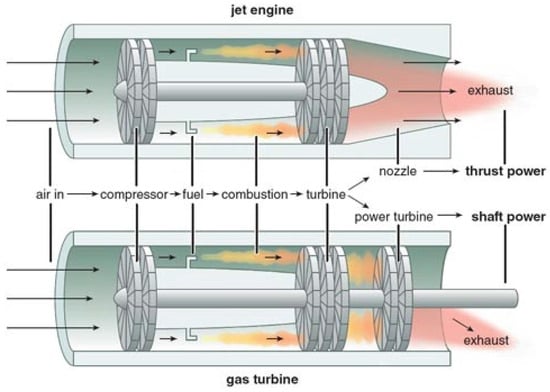
Figure 74.
Basic configuration of a jet engine (
top
) and stationary gas turbine (
Figure 5. Analysis of publication citations: leading authors in gas turbine and ammonia research. Data were generated using VOS-viewer [152].
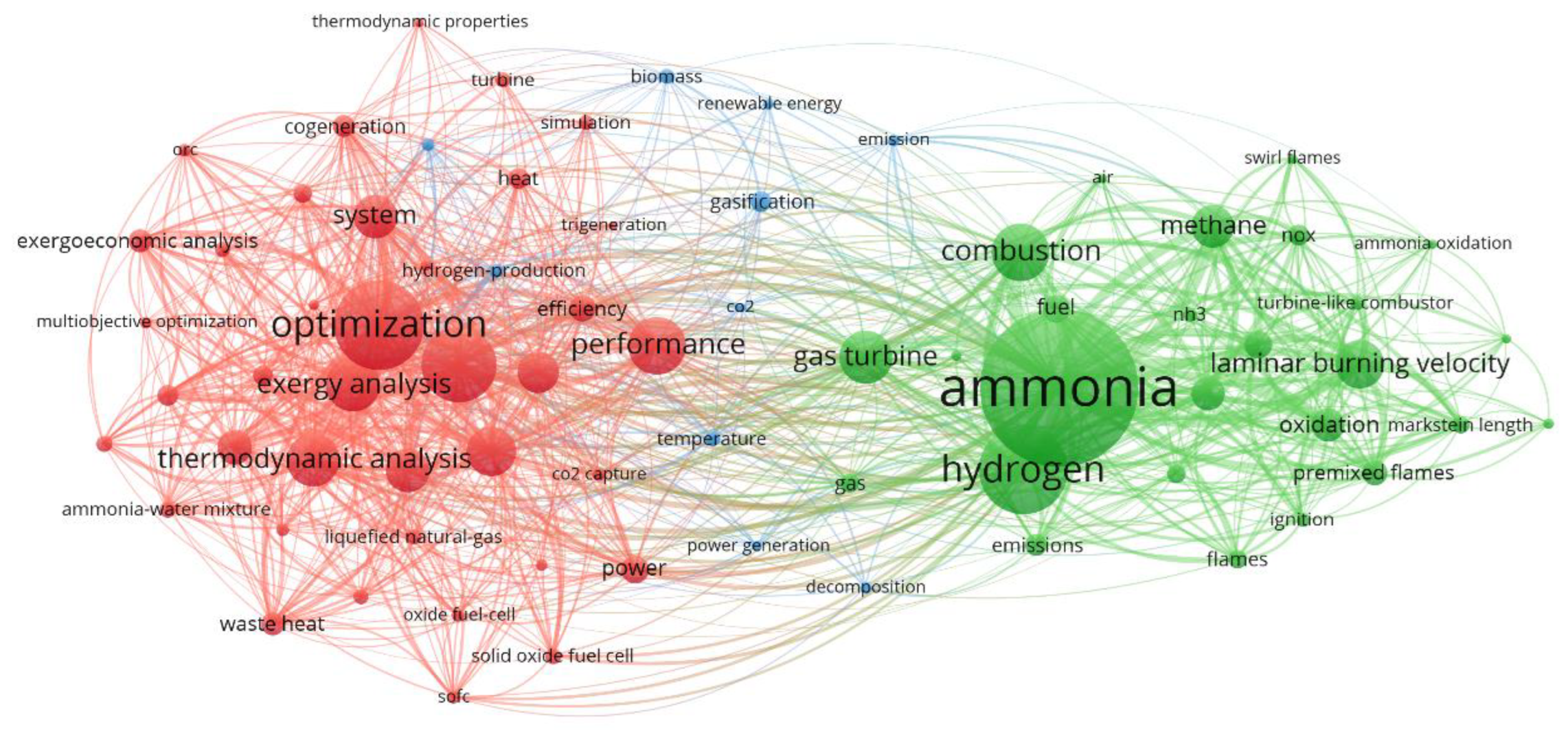
Figure 6. Keyword analysis: dominant terms in gas turbine and ammonia research publications. Data were generated using VOS-viewer [152].
The operation of gas turbines under severe operation conditions necessitates the development of new and innovative material technologies. Conventional gas turbines use a wide range of materials, from single-crystal nickel-based alloys for high-pressure turbine blades to polymer composites for fan blades in aero-engines [167][153]. The material specifications for heavily loaded aero-engine components are typically more demanding than those for stationary gas turbines, owing to tougher and more rapidly changing operating conditions. During the take-off and landing of an aircraft, for example, the output power of the gas turbine can shift dramatically in a matter of seconds, causing transient thermal loading and associated stress levels that encourage fatigue degradation in the materials. Emergency shutdowns pose the most challenging cycle circumstance for land-based turbines and can stop their long-term operation [167,168][153][154].
The demand for more flexible energy generation with quick gas turbine operation is also growing, particularly with the addition of more renewable energy sources to the electrical grid. As a result, the gap between the needs of aviation and stationary gas turbines may decrease, leading to an increased usage of aero-engine-derived technologies. Generally, the use of aero-engines has benefited stationary gas turbine technology [167,168][153][154]. Although aero-engines have been engineered to achieve higher turbine inlet temperatures, resulting in reduced fuel consumption and enhanced power generation capabilities that exceed those of land-based systems, this has an impact on the structural materials used in the high- and low-pressure turbine components, as well as the combustion chamber. In a modern aero-engine, the mechanical load due to centrifugal forces at full speed (12,000 rpm) on the blade root of a single blade in a modern aero-engine can be equal to the weight of a double-decker bus [169][155]. Additionally, thermal barrier coating systems used for heavily loaded engine components, such as initial blades and high-pressure turbine vanes, are often more strain-tolerant in aero-engines for both temperature and fatigue reasons. The choice of material in aero-engines is also influenced by weight, in addition to demanding operating conditions. When engine weight decreases, materials with high specific strength are developed, resulting in the use of titanium alloys in the compressor of aviation engines, such as Ti6Al4V or more sophisticated alloys with a maximum service temperature of 600 °C (1112 °F) [167][153].
Safety laws and regulations also impose additional material standards for aero-engines, such as the ability of fan housing to resist the failure of a fan blade. Carbon fibre-reinforced polymers are one of the ideal materials for this application [167][153]. The phenomenon of foreign object damage (FOD), caused by larger objects like birds, debris, engine components, misplaced tools, or hail being drawn into the turbine, can also damage blades. The Federal Aviation Administration (FAA) mandates that oxide ceramic textile firewall blankets used to separate a burning aero-engine from the rest of the aircraft should withstand 15 min at 1093 °C (1999 °F) without flame penetration [167][153].
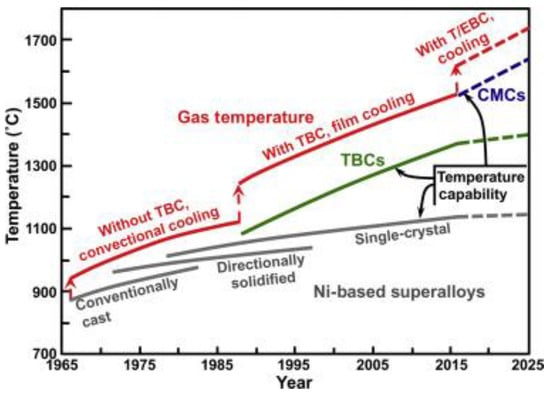
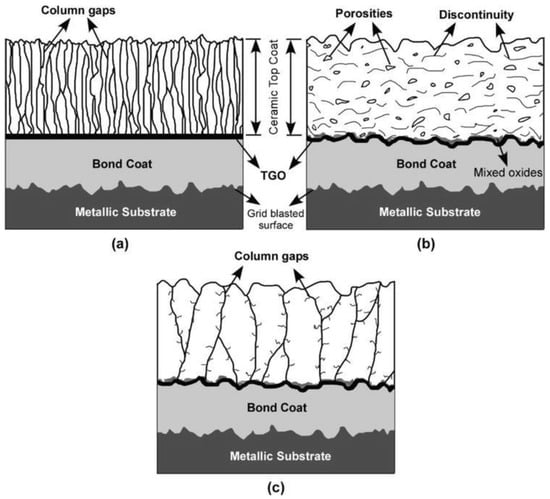
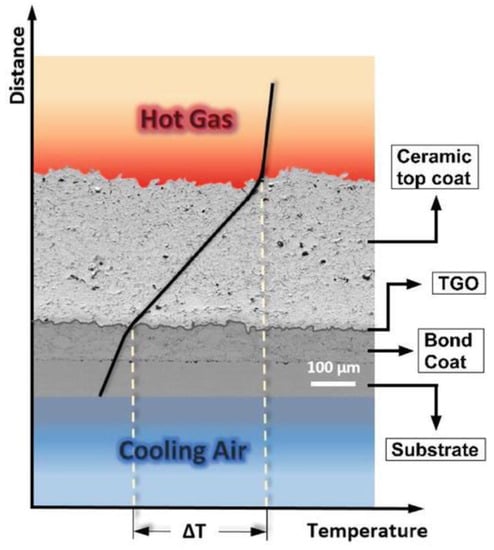
Coatings are an essential part of the design of gas turbines. They are applied to metallic structural materials to provide performance advantages that cannot be achieved with a single material. Without coatings, no single-crystal alloy with high strength and low creep would survive in a gas turbine. Similarly, no oxidation-resistant alloy can provide the necessary mechanical strength to the structural materials [168][154]. Figure 87 illustrates the progression and prediction of the temperature capabilities of materials used in gas turbine engines, including nickel-based superalloys, thermal barrier coatings (TBCs), ceramic matrix composites (CMCs), and thermal/environmental barrier coatings (T/EBCs), as well as the highest gas temperatures that can be tolerated with cooling (rough estimates) [167,168][153][154]. The figure depicts the development of temperature capabilities in material families over the past few decades. The saturation of single-crystal Ni-based alloys can be overcome using TBCs, which also allows for years of rapid advancement. The thermal/environmental barrier coatings (T/EBCs) that protect CMCs may withstand temperatures of more than 1500 °C (2732 °F) [167,168][153][154].
3.2. Challenges in Gas Turbines Fired on Ammonia–Hydrogen Fuels
Gas turbines that use ammonia and hydrogen as fuels face various challenges that need to be addressed to ensure reliable and efficient operation. Blending these fuels for gas turbines is a promising approach that could generate significant amounts of zero-carbon energy, thus contributing to mitigating climate change. However, ammonia and hydrogen fuels present several complexities compared with conventional fossil fuels [11][131]. They are highly reactive gases that can corrode and damage gas turbine components, particularly at high temperatures. The materials used in gas turbines should be capable of withstanding these corrosive gases and maintaining their structural integrity over time. Advanced materials, coatings, and surface treatments can help improve material compatibility [170][156]. Furthermore, ammonia and hydrogen have different storage and transportation requirements compared with other conventional fossil fuels. Ammonia requires specialised tanks and pipelines that are resistant to corrosion and can maintain high fuel pressure, whereas hydrogen requires storage at high pressure and low temperature. Both fuels require careful handling and transportation to ensure safety and prevent leaks or accidents. [2,171][2][157]. Figure 98 emphasises the critical material-related issues such as high-temperature oxidation, corrosion, hydrogen embrittlement, and thermal barrier coatings that need to be addressed in the design and development of gas turbine systems.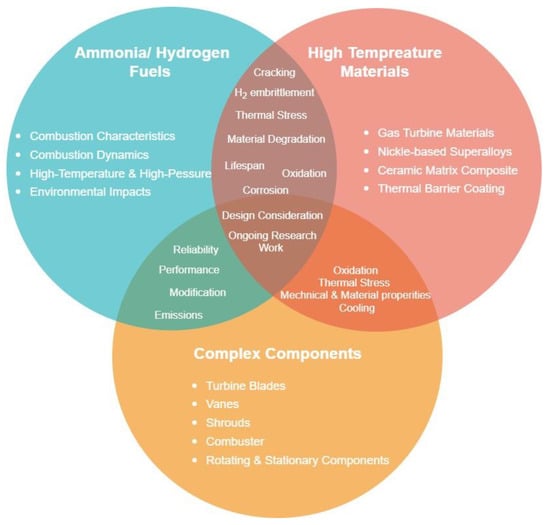
Figure 98.
Material challenges in ammonia/hydrogen-fuelled gas turbines.
3.3. High-Temperature Materials for Complex Components
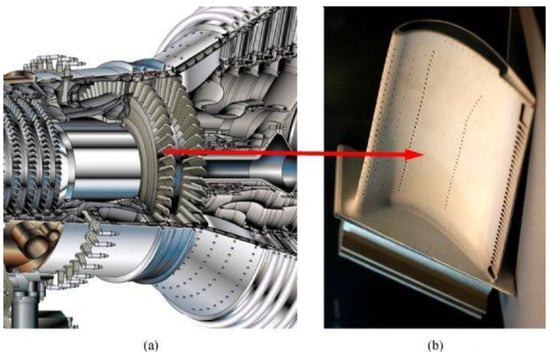
Materials selection might be problematic for gas turbines due to extreme environmental conditions. Cryogenic liquids cause severe toughness and ductility problems with metals, while metals that encounter hot exhaust gases should withstand creep and stress rupture at high temperatures [185][171]. Various components of a gas turbine require materials with high mechanical, thermal, and manufacturability properties, as well as stability under working conditions [168][154]. The thermodynamic cycle determines the gas temperature and pressure, and thus assists in materials selection for each section, from the fan at the front to the compressor, combustor, and turbine. Gas turbines mostly have two types of axial turbines: impulse turbines and reaction turbines. The full enthalpy drop between the nozzle and the rotor causes a very high velocity to enter the rotor of an impulse turbine, resulting in a high enthalpy drop. The enthalpy drop is divided between the rotor and the nozzle in the reaction turbine. The fan’s blades are primarily made of titanium alloys and polymer matrix composites, with some aluminium in outer, static structural components. In the compressor, the gas-stream temperature can rise as high as 700 °C (1292 °F) under compression, and the blades and disks are mostly titanium alloy [166][151]. High-temperature nickel- and cobalt-based sheet alloys have been the main materials used in the combustor section. Figure 11 0 shows an example of the main parts of the Alstom gas turbine and the materials used in each part [168][154].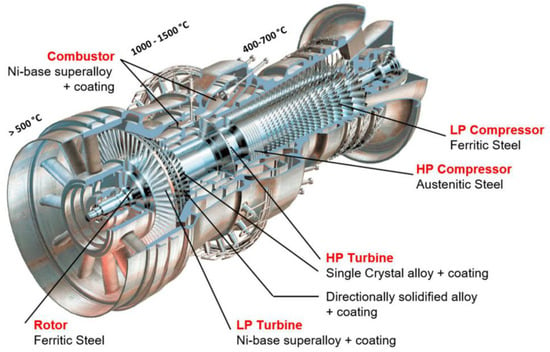
Figure 110. Main parts of an Alstom gas turbine, exposure conditions and materials used in different sections [168].
Main parts of an Alstom gas turbine, exposure conditions and materials used in different sections [154].
-
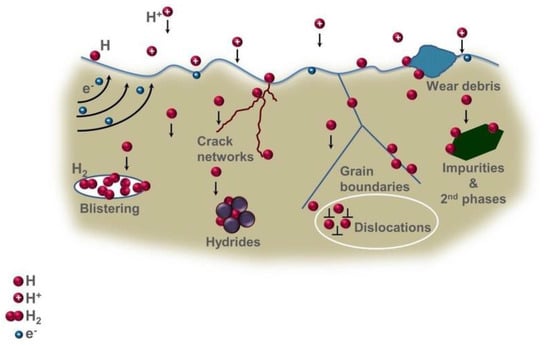
Hydrogen embrittlement can occur through different mechanisms, including stress corrosion cracking, hydrogen-induced cracking, or hydrogen embrittlement [188][174]. The diffusion of hydrogen atoms into a metal can make it more brittle and prone to cracking. This process can cause various metals, especially high-strength steel, to become brittle and fracture following exposure to hydrogen [187][173].
-
Hydrogen embrittlement can cause material degradation and reduced efficiency in gas turbine engines [189][175]. This phenomenon can lead to cracking, blistering, and other forms of damage to the material [190][176]. The use of hydrogen as a fuel in gas turbines can also increase the turbine inlet temperature, which can lead to material degradation and reduced efficiency [189][175].
-
To avoid the degradation of turbine performance when using hydrogen in combustion, the system may require some changes, such as varying the mass flow rate, changing the pressure ratio, or the design and structure of the cycle [188][174]. The use of hydrogen can also require changes in the gas turbine design to avoid material degradation and maintain performance. Materials can also be designed to be more resistant to hydrogen embrittlement [188][174].
-
There is ongoing research being conducted to better understand the effects of hydrogen embrittlement on materials used in gas turbine engines and how to mitigate these effects [189,[175][176]. Studies have investigated the effect of adding hydrogen to natural gas on combustion using numerical simulation [190][176].
Table 21.
Materials used in gas turbines and rocket engines.
| Ref. | Material | Examples | Applications | Temp. Range | Remarks |
|---|---|---|---|---|---|
| [185,192][171][178] | Austenitic stainless steels | 316, 321, 347, 21-6-9, 16-25-6 | Nozzle tubing, ducts, bolts, bellows, hydraulic tubing, washers, shims, turbine discs, injectors, compressor | −423 °F to 600 °F | Susceptible to pitting and stress corrosion, low cost, and high strength |
| [185,192][171 |
Figure 132.
(
a
) High-pressure turbine rotor assembled with (
| Class | Alloy | Compositions (wt.%) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Co | Mo | W | Al | Ti | Ta | Nb | Re | Ru | Hf | C | B | Zr | Ni | |||||||
| Conventional Cast (CC) | IN-713LC | 12 | - | 4.5 | - | 5.9 | 0.6 | - | 2 | - | - | - | 0.05 | 0.01 | 0.1 | Bal | |||||
| ][178] | Martensitic stainless steels | 440c | Bearings–balls, races | −423 °F to 300 °F | Susceptible to all forms of corrosion and low cost | ||||||||||||||||
| IN-738LC | 16 | 8.5 | 1.75 | 2.6 | 3.4 | 3.4 | 1.75 | 0.9 | - | - | - | 0.11 | 0.01 | 0.04 | Bal | [185][171] | PH stainless steels | 17-4 PH, 17-7 PH, 15-5 PH | Valve parts–stems, poppets | 110 °F to 200 °F |
Susceptible to H |
| René 80 | 14 | 9 | 4 | 4 | 2 embrittlement., stress corrodes in high-strength temperatures, marginal for cryogenic applications |
||||||||||||||||
| 3 | [168,185,186,192][154][171][172][178] | Nickle-based superalloys | 718, 625, WASPALOV®®, MAR-M-246 and 247®® | ||||||||||||||||||
| 154 | |||||||||||||||||||||
| ] | |||||||||||||||||||||
| [171][178] | Titanium alloys | Ti-5AI-2.5 Sn ELI, Ti-6AI-4V ELI, Ti-6AI-6V-2Sn, Ti-10Y-2Fe-3AI | Impellers, inducers, pump housings, valve bodies, ducts, gimbal blocks, pressure bottles, hydraulic tubing compressor |
−423 °F to 600 °F | Pyrophoric reaction in LOX, pure GOX, red fuming nitric acid, may absorb hydrogen above -110 °F, low density, high strength, high stiffness, high cost, poor ductility, and excellent oxidation resistance | ||||||||||||||||
| 4.7 | - | - | - | - | 0.8 | 0.16 | 0.015 | 0.01 | Bal | , HASTELLOY-C®® Incoloy®® 783, Haynes®® 242®® |
Impellers, inducers, pump housings, valves, ducts, manifolds, bolts, turbine blades, turbine discs, shafts, bellows, stators, injectors, combustors, vanes |
−423 °F to 1500 °F | Susceptible to hydrogen environment embrittlement, high strength, high cost, creep at high temperature and dimensional stability (for some alloys) | ||||||||
| [185,192][171][178] | Iron-based superalloys | 903, 909, A286 | Struts, ducts, bellows, bolts, turbine discs | −423 °F to 1100 °F | Resistant to hydrogen environment, embrittlement, high strength, limited oxidation resistance | ||||||||||||||||
| [185][171] | Aluminium alloys | A356, A357, 6061, 7075, T73, 2219 | Pump housings, impellers, injectors, gear cases, brackets, valve bodies | −423 °F to 200 °F | Often used as castings | ||||||||||||||||
| [185][171] | Copper alloys | OFHC Cu, NARLloy-Z, NARloy-A | Thrust chambers, injector rings, baffles | −423 °F to 1000 °F | High oxygen grades, susceptible to hydrogen reaction embrittlement |
||||||||||||||||
| [168,185,192][ | |||||||||||||||||||||
3.4. Turbine Blades: Design, Heat Flux, and Cooling Technology
The process of designing an aerofoil for gas turbine blades is a complex task that requires expertise in several fields, including aerodynamics, materials science, and manufacturing. The general steps involved in designing an aerofoil for gas turbine blades include:-
Determining the operating conditions of the gas turbine such as the air flow rate, temperature, pressure, and Mach number. The Mach number is defined as the ratio of velocity to the acoustic speed of a gas at a given temperature M = V/a, where (V) is the gas velocity and (a) is the acoustic speed. The acoustic speed is the ratio change in pressure of the gas with respect to its density if the entropy is held constant [12][191].
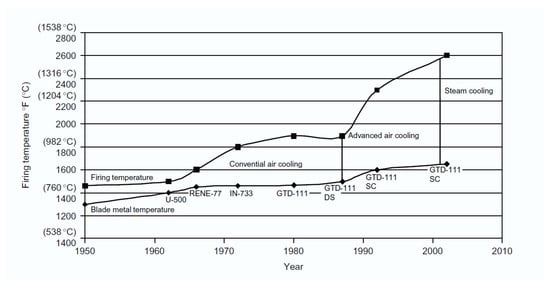

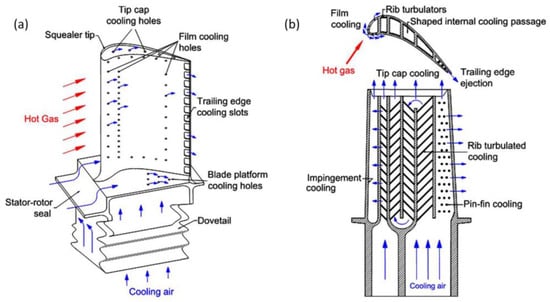
Figure 198.
Gas turbine blade cooling schematic showing (
a
) external cooling and (
Table 43. Different types of gas turbine technologies. GT: gas turbine; Exp: experimental; Sim: simulation; Calc: calculation/modelling; TIT: turbine inlet temperature (°C).
| Ref. | Year | Research Type | Turbine Type |
Working Fuel | TIT (°C) |
|---|
.
Various types of microscopes used for materials characterisation.
| Techniques | Advantage | Disadvantage | Remarks | Power Capacity | Cycle Efficiency | GT Materials | Remarks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [212,213][199][200] | |||||||||||||||
| Scanning electron microscopy (SEM) | 1989 | Exp. | Alstom’s GT24 | The ability to capture high-resolution images of the surface and subsurface characteristics of materials, identification of crystallographic orientation and grain boundaries, and analysis of elemental composition and chemical bonding. Additionally, SEM is user-friendly and easy to operate with proper training and advances in computer technology and associated software [225,226,227][212][213][214]. | It is expensive and requires a vacuum environment, which can limit the analysis of certain materials. Additionally, SEM can be sensitive to charging effects, which can affect image quality. Sample preparation for SEM can be time-consuming and requires specialised equipment [227, | N. G | 228 | 1093 °C (1999 °F) | 188 MW | ][214][215]. | This technique can be used to analyse the microstructure of gas turbine blade materials, including the crystallographic orientation and grain boundaries. It can also be utilised to study the surface characteristics of the material such as wear, corrosion, and cracks [225, | 36.9% | 226 | Combustor—Ni-based superalloy + coating. Blades—Single crystal alloy + coating. | Superior part load efficiencies. Low emissions from 40% to 100% load. High fuel flexibility (natural gas composition; oil). Very low combined cycle start-up times. |
| , | 229 | , | 230][212][213][216][217]. | [183,214][169][201] | 1987 | ||||||||||
| Transmission electron microscopy (TEM) | It provides high-resolution imaging of the microstructure and crystal defects. It can be used to identify the crystallographic orientation and grain boundaries. Also, it can be used to analyse the elemental composition and chemical bonding of materials [231] | Calc. | [218]. | It requires a vacuum environment, which can limit the analysis of certain materials. TEM is sensitive to radiation, which can affect image quality. Sample preparation can be time-consuming and require specialised equipment [231 | Allison 501-KB | Air | |||||||||
| 1950 | |||||||||||||||
| Exp. | Rolls-Royce Avon 200 | Kerosene | 1700 °C/1150 °C (3092 °F/2102 °F) | 17 MW | 27.6% | Blades | are made from single-crystal alloy + coating | In 2007, the gas turbine was improved by upgrading and coating the material used for the turbine blades, as well as changing the blade material to a single crystal and redesigning them to improve thermal efficiency and cycle performance. Swirler burner technology was also implemented in the combustion system to reduce combustion instability and emissions. | |||||||
| [212][199] | 1998 | Exp. | GE 9H | N. G | 1430 °C (2606 °F) | 480 MW | 60% | Blades are made from single-crystal alloy + coating | The turbine blades are cooled using steam instead of air for better cooling effectiveness and higher heat capacity. There are no detrimental effects of steam on the properties of the coated single-crystal alloy, and there are no mechanical or thermal effects. The machine will be highly instrumented and stripped down. |
3.5. Materials Characterisation Techniques
Materials characterisation techniques are essential for the development and optimisation of gas turbine materials and blades. These techniques are used to investigate the structural, mechanical, and physical properties of materials and components, enabling the identification of any defects and the optimisation of performance [217,218][204][205]. The following are some of the commonly used materials characterisation techniques for gas turbine materials and blades:-
Optical microscopy is a widely used method for characterising the microstructure of materials. It provides a large field of view and high depth of field, making it ideal for imaging larger features. In the case of gas turbine materials and blades, optical microscopy can be used to assess the quality of the material and identify any defects such as cracks or voids [219][206].
-
Scanning electron microscopy (SEM) is a high-resolution imaging technique that is used to investigate the surface morphology and composition of materials. It is particularly useful for investigating the microstructure of gas turbine materials and blades, as well as identifying any defects or degradation of the blade surfaces [220][207].
| ] | |||||||||||||
| [ | 218 | ]. | 982 °C (1800 °F) | 3.4 MW | 24.0% | Combustor—Hastelloy X (AMS.5536). Blades—Inconel 738+ coating | TEM can be used to examine the crystal structure and defects within turbine blade materials, such as dislocations, vacancies, and interstitials | The study found that supplying extra air at the required temperature increased the mass flow through the turbine, resulting in increased efficiency and power output. However, creating steam for injection by heating it in the combustor reduced the efficiency. The characteristics of the working fuel were found to be one of the most important factors in increasing output. | |||||
| [ | 230 | ] | [217]. | [212,214][199][201] | 1982 | Exp. | Allison 501-KB5 | N. G | 1035 °C (1895 °F) | 3.9 MW | 29.5% | Combustor—Hastelloy X (AMS.5536). Blades—Mar-M-246, AEP 32 coating | The 501-KB engine was upgraded by increasing the engine speed, modifying the exhaust diffuser, and increasing the firing temperature by a specific amount. The vane and blade materials were changed, and the coating was modified to ensure consistent structural life without any changes to the aerofoil design. |
| [7,212][ | |||||||||||||
| Atomic force microscopy (AFM) | It provides high-resolution imaging of surface topography and features. Used to analyse surface roughness, wear, and corrosion and measure mechanical properties such as surface adhesion and elasticity [225,232,233][212][219][220]. | Limited to analysing surfaces in air or liquid environments, which may not be representative of operating conditions. It can be affected by tip wear and contamination, which can affect image quality and accuracy. A limited depth penetration makes it less useful for analysing subsurface features [232,[219233]][220]. |
AFM can be used to examine the surface roughness and mechanical properties of turbine blade materials, including hardness, elasticity, and adhesion. It can provide information on the topography and morphology of materials at the nanoscale [225,234][212][221]. | 7][199] | 1971 | ||||||||
| Laser scanning confocal microscopy (LSCM) | |||||||||||||
| It provides high-resolution images compared with optical microscopy. LSCM is a non-destructive imaging technique, which means it can be used to study samples without altering or damaging them. Used to create three-dimensional images of samples, which is useful for studying the structure and morphology of biological specimens. LSCM can be used to study a wide range of materials, including metals, ceramics, and polymers, as well as biological samples | [241,242][228][229]. | It can be expensive to purchase and maintain, which can be a limitation for smaller labs or research groups. Requires careful sample preparation and staining, which can be time-consuming and may affect the quality of the image. LSCM has a limited field of view, which means that larger samples may need to be imaged in multiple parts and stitched together, which can introduce errors [242][229]. | LSCM can be used to examine the surface topography and roughness of turbine blade materials at high resolution [243][230 | ||||||||||
| [185][171] | Beryllium | Be-98, BeO-1.5 | Small thrust chambers | 70 °F to 1200 °F | Brittle, avoid all notches in design, hazardous material, not weldable | ||||||||
| [185][171] | Cobalt alloys | HAYNES 188, L-605, ELGILOY, MP 3Sn, STELLITE 21 | Injector posts, ducts, springs, turbine blades, combustor |
320 °F to 2100 °F | Vary in susceptibility to hydrogen environment embrittlement | ||||||||
| [185][171] | Low-alloy steels | 4130, 4340, 9310, 52,100 | Thrust mounts, frames, reinforcing bands, gears, shafts, bolts, bearings | 70 °F to 300 °F | Susceptible to corrosion, marginal for cryogenic applications | ||||||||
| Exp. | [185][171] | Fluorocarbon polymers | Kel-F, PTFE, FEP | Seals, coatings, rub rings, electrical insulation | −423 °F to 200 °F | Generally compatible with liquid oxygen | |||||||
| [185][171] | Elastomers | Nitrile rubber, silicone rubber, chloroprene rubber, butyl rubber, fluorocarbon rubber | O-rings, gaskets, sealants, electrical insulation, adhesives | 70 °F to 300 °F | Not compatible with liquid oxygen | ||||||||
| [185][171] | Carbon | P5N, P692 | Combustion chamber throat inserts, dynamic turbine seals | −423 °F to 600 °F | Brittle material | ||||||||
| [163,167,168,185,186,192][148][153][154][171][172][178] | Ceramics | Al2O3, Zro, WC, Sio2 | Protective coatings on turbine blades, nozzles, thrust chambers, thermal insulation, valve seat, Poppet coatings | −423 °F to 1500 °F | High temperatures, brittle materials, low density, high specific strength, poor fracture toughness and poor ductility |
| Mar-M247 | |||||||||||||||
| 8 | 10 | 0.6 | 10 | 5.5 | 1 | 3 | - | - | - | 1.5 | 0.15 | 0.015 | 0.03 | Pratt & Whitney | Bal |
| JT8D-15A | Kerosene | 1004 °C (1839 °F) | 25 MW | 40% | The combustor section and blades are made from nickel-based superalloys | ||||||||||
| X-Ray diffraction (XRD) | Compared with other gas turbines, the JTBD-15A has a high bypass ratio, resulting in a greater amount of air being directed through the engine to produce thrust rather than being lost as waste heat. | ||||||||||||||
| It provides information about the crystal structure and phase composition of materials. Used to analyse the degree of crystallographic orientation in polycrystalline materials. The non-destructive technique can be used on bulk samples | [235,236][222][223]. | It is sensitive to sample size and homogeneity, which can affect analysis accuracy. Requires knowledge of the crystal structure and phase composition of the material being analysed. Difficulties in providing detailed information about microstructure or surface features [235,236][222][223]. |
XRD can be used to examine the crystal structure of turbine blade materials and identify the presence of different phases or crystallographic defects [226][213]. | DS | 1st | Mar- | [215 M200Hf |
8 | 9 | ,216][202][203- | 12 | 5 | 1.9 | - | ] |
| Optical microscopy | |||||||||||||||||||||||
| It is relatively inexpensive compared with other imaging techniques. Easy to use. Has a larger field of view compared with LSCM, which allows larger samples to be imaged without the need for stitching. Widely used in biological research and can also be used to study materials, such as metals and polymers | |||||||||||||||||||||||
| [ | 1 | 237 | - | - | ,238][224][225]. | It provides lower-resolution images compared with LSCM, which can make it difficult to see fine details. It can be destructive, especially if the sample needs to be stained or sectioned. It has a limited depth of field, which can make it difficult to image samples with a large height or depth [238][225]. | Optical microscopy can be used to examine the surface and subsurface features of turbine blade materials, including surface roughness, grain size, and cracks [225,226][212][213]. | 2 | 0.13 | 0.015 | 0.03 | Bal | |||||||||||
| CM247LC | 8.1 | ||||||||||||||||||||||
| Scanning transmission electron microscopy (STEM) | 9.2 | It provides high-resolution imaging of surface topography and features. Used to analyse crystal structure, defects, and chemical composition at the atomic level. Also used to analyse thin films and bulk materials [239][226]. | Requires a vacuum environment, which can limit the analysis of certain materials. High-resolution imaging requires careful sample preparation and may damage the sample. Limited field of view, making it less useful for analysing large areas or volumes [239][226]. | 0.5 | 9.5 | 5.6 | 0.7 | 3.2 | - | - | - | 1.4 | STEM can be used to examine the crystal structure and chemical composition of turbine blade materials at an atomic resolution | 0.07 | [240] | 0.015 | [227] | 0.007 | Bal | ||||
| . | 2nd | CM186LC | |||||||||||||||||||||
| Energy-dispersive X-Ray spectroscopy (EDS) | It provides information about the elemental composition and distribution of materials. Used to analyse small sample volumes or areas. Used in conjunction with other microscopy techniques to provide additional information [226, | 6 | 228][213][215] | 9.3 | . | Affected by variations in sample thickness, crystal structure and beam penetration depth. Spectral interference can occur when multiple elements have overlapping X-ray spectra. May not provide detailed information about microstructure or surface features [228][215 | 0.5 | ]. | EDS can be used to analyse the chemical composition and elemental distribution of turbine blade materials [225,226 | 8.4 | 5.7 | 0.7 | 3.4 | - | 3.0 | ][212 | - | ][213 | 1.4 | 0.07 | 0.015 | 0.005 | Bal |
| ] | PWA1426 | 6.5 | 10 | 1.7 | ] | 6.5 | . | 6 | - | 4 | - | 3.0 | - | 1.5 | 0.1 | 0.015 | 0.1 | Bal | |||||
| SC | 1st | CMSX-2 | 8 | 5 | 0.6 | 8 | 5.6 | 1 | 6 | - | - | - | - | - | - | - | Bal | ||||||
| PWA1480 | 10 | 5 | - | 4 | 5 | 1.5 | 12 | - | - | - | - | - | - | - | Bal | ||||||||
| René N4 | 9 | 8 | 2 | 6 | 3.7 | 4.2 | 4 | 0.5 | - | - | - | - | - | - | Bal | ||||||||
| AM1 | 7 | 8 | 2 | 5 | 5 | 1.8 | 8 | 1 | - | - | - | - | - | - | Bal | ||||||||
| RR2000 | 10 | 15 | 3 | - | 5.5 | 4 | - | - | - | - | - | - | - | - | Bal | ||||||||
| 2nd | CMSX-4 | 6.5 | 9.6 | 0.6 | 6.4 | 5.6 | 1 | 6.5 | - | 3 | - | 0.1 | - | - | - | Bal | |||||||
| PWA1484 | 5 | 10 | 2 | 6 | 5.6 | - | 9 | - | 3 | - | 0.1 | - | - | - | Bal | ||||||||
| René N5 | 7 | 8 | 2 | 5 | 6.2 | - | 7 | - | 3 | - | 0.2 | - | - | - | Bal | ||||||||
| 3rd | CMSX-10 | 2 | 3 | 0.4 | 5 | 5.7 | 0.2 | 8 | - | 6 | - | 0.03 | - | - | - | Bal | |||||||
| 4th | TMS-138 | 2.9 | 5.9 | 2.9 | 5.9 | 5.9 | - | 5.6 | - | 4.9 | 2 | 0.1 | - | - | - | Bal | |||||||
| 5th | TMS-162 | 2.9 | 5.8 | 3.9 | 5.8 | 5.8 | - | 5.6 | - | 4.9 | 6 | 0.09 | - | - | - | Bal | |||||||
| Re-free | CMSX-7 | 6 | 10 | 0.6 | 9 | 5.7 | 0.8 | 9 | - | - | - | 0.2 | - | - | - | Bal | |||||||
| Low Re | CMSX-8 | 5.4 | 10 | 0.6 | 8 | 5.7 | 0.7 | 8 | - | 1.5 | - | 0.1 | - | - | - | Bal |
- ]
- Atomic force microscopy (AFM) is a technique that provides high-resolution imaging of surfaces at the nanoscale. It is particularly useful for assessing the degradation of the blade surfaces, enabling the identification of any defects such as pitting, cracking or corrosion
| . |
- Energy-dispersive X-ray spectroscopy (EDS) is a technique that is used to obtain the chemical composition of materials. In the case of gas turbine materials and blades, EDS analysis can be used to identify the presence of impurities or degradation products, enabling the identification of any defects or degradation mechanisms
- [
Table 54
References
- Sartbaeva, A.; Kuznetsov, V.; Wells, S.A.; Edwards, P. Hydrogen nexus in a sustainable energy future. Energy Environ. Sci. 2008, 1, 79–85.
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.; Bowen, P. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102.
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/air premixed flames at various pressures. Fuel 2015, 159, 98–106.
- Chu, H.; Xiang, L.; Nie, X.; Ya, Y.; Gu, M.; Jiaqiang, E. Laminar burning velocity and pollutant emissions of the gasoline components and its surrogate fuels: A review. Fuel 2020, 269, 117451.
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines. J. Clean. Prod. 2021, 296, 126562.
- Yapicioglu, A.; Dincer, I. Experimental investigation and evaluation of using ammonia and gasoline fuel blends for power generators. Appl. Therm. Eng. 2019, 154, 1–8.
- Gaffin, W. NASA ECI Programs: Benefits to Pratt & Whitney Engines. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 1982.
- Evans, M.J.; Chinnici, A.; Medwell, P.R.; Dally, B.B. Autoignition of Hydrogen/Ammonia Blends at Elevated Pressures and Temperatures. 2019. Available online: https://www.h2knowledgecentre.com/content/conference902 (accessed on 20 August 2023).
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Modeling combustion of ammonia/hydrogen fuel blends under gas turbine conditions. Energy Fuels 2017, 31, 8631–8642.
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133.
- Breeze, P. Gas-Turbine Power Generation; Academic Press: Cambridge, MA, USA, 2016.
- Elmanakhly, F. Co-Production of Hydrogen and Ethylene in an Oxygen Permeable Membrane Reactor; University of Waterloo: Waterloo, ON, Canada, 2022.
- Taamallah, S.; Vogiatzaki, K.; Alzahrani, F.M.; Mokheimer, E.M.; Habib, M.; Ghoniem, A.F. Fuel flexibility, stability and emissions in premixed hydrogen-rich gas turbine combustion: Technology, fundamentals, and numerical simulations. Appl. Energy 2015, 154, 1020–1047.
- Bothien, M.R.; Ciani, A.; Wood, J.P.; Fruechtel, G. Toward decarbonized power generation with gas turbines by using sequential combustion for burning hydrogen. J. Eng. Gas Turbines Power 2019, 141, 121013.
- Andersson, M.; Larfeldt, J.; Larsson, A. Co-Firing with Hydrogen in Industrial Gas Turbines; Svenskt Gastekniskt Center: Malmö, Sweden, 2013.
- Bancalari, E.; Chan, P.; Diakunchak, I.S. Advanced hydrogen gas turbine development program. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2007.
- Wu, J.; Brown, P.; Diakunchak, I.; Gulati, A.; Lenze, M.; Koestlin, B. Advanced gas turbine combustion system development for high hydrogen fuels. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2007.
- Neugebauer, R. Hydrogen Technologies; Springer Nature: Berlin, Germany, 2023.
- Ebrahimi, H. Overview of gas turbine augmentor design, operation, and combustion oscillation. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9–12 July 2006.
- Bohan, K.; Klapdor, E.; Prade, B.; Haeggmark, A.; Bulat, G.; Prasad, N.; Welch, M.; Adamsson, P.; Johnke, T. Hydrogen Power with Siemens Gas Turbines. A Siemens White Paper, 2020 Hydrogen Power with Siemens Gas Turbines. Available online: https://www.siemens-energy.com/csc (accessed on 1 April 2020).
- Jones, R.; Goldmeer, J.; Monetti, B. Addressing gas turbine fuel flexibility. GE Energy 2011, 4601, 1–20.
- Rahm, S.; Goldmeer, J.; Molière, M.; Eranki, A. Addressing gas turbine fuel flexibility. In Proceedings of the POWER-GEN Middle East Conference, Manama, Bahrain, 17–19 February 2009.
- Ditaranto, M.; Heggset, T.; Berstad, D. Concept of hydrogen fired gas turbine cycle with exhaust gas recirculation: Assessment of process performance. Energy 2020, 192, 116646.
- Hormaza Mejia, N.A. Experimental Investigation of Hydrogen and Hydrogen/Methane Mixture Leakage from Low-Pressure Natural Gas Infrastructure. Master’s Thesis, University of California, Irvine, CA, USA, 2019.
- Chiesa, P.; Lozza, G.; Mazzocchi, L. Using hydrogen as gas turbine fuel. J. Eng. Gas Turbines Power 2005, 127, 73–80.
- Mehrpooya, M.; Sharifzadeh, M.M.M.; Katooli, M.H. Thermodynamic analysis of integrated LNG regasification process configurations. Prog. Energy Combust. Sci. 2018, 69, 1–27.
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254.
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233.
- Choi, S.; Lee, S.; Kwon, O.C. Extinction limits and structure of counterflow nonpremixed hydrogen-doped ammonia/air flames at elevated temperatures. Energy 2015, 85, 503–510.
- Li, J.; Huang, H.; Deng, L.; He, Z.; Osaka, Y.; Kobayashi, N. Effect of hydrogen addition on combustion and heat release characteristics of ammonia flame. Energy 2019, 175, 604–617.
- Ayed, A.H.; Kusterer, K.; Funke, H.-W.; Keinz, J.; Striegan, C.; Bohn, D. Experimental and numerical investigations of the dry-low-NOx hydrogen micromix combustion chamber of an industrial gas turbine. Propuls. Power Res. 2015, 4, 123–131.
- Goldmeer, J.; Catillaz, J. Hydrogen for Power Generation. General Electric. 2021. Available online: www.ge.com/gas-power/future-of-energies (accessed on 1 March 2022).
- Meziane, S.; Bentebbiche, A. Numerical study of blended fuel natural gas-hydrogen combustion in rich/quench/lean combustor of a micro gas turbine. Int. J. Hydrogen Energy 2019, 44, 15610–15621.
- Park, S.; Kim, U.; Lee, M.; Kim, S.; Cha, D. The effects and characteristics of hydrogen in SNG on gas turbine combustion using a diffusion type combustor. Int. J. Hydrogen Energy 2013, 38, 12847–12855.
- Noble, D.; Wu, D.; Emerson, B.; Sheppard, S.; Lieuwen, T.; Angello, L. Assessment of current capabilities and near-term availability of hydrogen-fired gas turbines considering a low-carbon future. J. Eng. Gas Turbines Power 2021, 143, 041002.
- Pasquariello, R. Gas Turbine Innovation, with or Without Hydrogen. Turbomachinery Magazine. 2020. Available online: https://www.turbomachinerymag.com/view/gas-turbine-innovation-with-or-without-hydrogen (accessed on 21 November 2021).
- Xing, F.; Kumar, A.; Huang, Y.; Chan, S.; Ruan, C.; Gu, S.; Fan, X. Flameless combustion with liquid fuel: A review focusing on fundamentals and gas turbine application. Appl. Energy 2017, 193, 28–51.
- Ishaq, H.; Dincer, I. A comprehensive study on using new hydrogen-natural gas and ammonia-natural gas blends for better performance. J. Nat. Gas Sci. Eng. 2020, 81, 103362.
- Arsalis, A. Thermodynamic modeling and parametric study of a small-scale natural gas/hydrogen-fueled gas turbine system for decentralized applications. Sustain. Energy Technol. Assess. 2019, 36, 100560.
- Koç, Y.; Yağlı, H.; Görgülü, A.; Koc, A. Analysing the performance, fuel cost and emission parameters of the 50 MW simple and recuperative gas turbine cycles using natural gas and hydrogen as fuel. Int. J. Hydrogen Energy 2020, 45, 22138–22147.
- De Robbio, R. Innovative combustion analysis of a micro-gas turbine burner supplied with hydrogen-natural gas mixtures. Energy Procedia 2017, 126, 858–866.
- Ciani, A.; Wood, J.P.; Wickström, A.; Rørtveit, G.J.; Steeneveldt, R.; Pettersen, J.; Wortmann, N.; Bothien, M.R. Sequential combustion in ansaldo energia gas turbines: The technology enabler for co2-free, highly efficient power production based on hydrogen. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2020.
- Power, G.S.L.C.D. Net Zero North West Cluster Plan. 2022. Available online: https://www.ukri.org/who-we-are/how-we-are-doing/research-outcomes-and-impact/innovate-uk/net-zero-north-west-cluster-plan/ (accessed on 20 August 2023).
- Patel, S. GE Secures First HA-Class Hydrogen Gas Power Deal: Long Ridge Energy Terminal. Power. 2020. Available online: https://www.powermag.com/ge-secures-first-ha-class-hydrogen-gas-power-deal-long-ridge-energy-terminal/ (accessed on 13 October 2020).
- Sundén, B. Hydrogen, Batteries and Fuel Cells; Academic Press: Cambridge, MA, USA, 2019.
- Langston, L.S. Generating a Greener Future: Combined cycle gas turbines are advancing electrical energy production. Am. Sci. 2021, 109, 80–84.
- Mati, A.; Ademollo, A.; Carcasci, C. Assessment of paper industry decarbonization potential via hydrogen in a multi-energy system scenario: A case study. Smart Energy 2023, 11, 100114.
- Erdener, B.C.; Sergi, B.; Guerra, O.J.; Chueca, A.L.; Pambour, K.; Brancucci, C.; Hodge, B.-M. A review of technical and regulatory limits for hydrogen blending in natural gas pipelines. Int. J. Hydrogen Energy 2023, 48, 5595–5617.
- Rahman, M.N.; Wahid, M.A. Renewable-based zero-carbon fuels for the use of power generation: A case study in Malaysia supported by updated developments worldwide. Energy Rep. 2021, 7, 1986–2020.
- Engstam, L. Power-to-X-to-Power in Combined Cycle Power Plants: A Techno-Economic Feasibility Study. 2021. Available online: http://kth.diva-portal.org/smash/record.jsf?pid=diva2%3A1605535&dswid=-9534 (accessed on 20 August 2023).
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.; Dedoussi, I.; De Joannon, M.; Fernandes, R.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S. Review on ammonia as a potential fuel: From synthesis to economics. Energy Fuels 2021, 35, 6964–7029.
- Statista. Production Capacity of Ammonia Worldwide from 2018 to 2021, with a Forecast for 2026 and 2030. Available online: https://www.statista.com/statistics/1065865/ammonia-production-capacity-globally/ (accessed on 20 August 2023).
- Aziz, M.; TriWijayanta, A.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062.
- Chavando, J.A.M.; Silva, V.B.; da Cruz Tarelho, L.A.; Cardoso, J.S.; Hall, M.J.; Eusébio, D. Chapter 7—Ammonia as an alternative. In Combustion Chemistry and the Carbon Neutral Future; Brezinsky, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 179–208.
- Lim, D.; Moon, J.A.; Yoon, C.W.; Lim, H. Feasibility of electricity generation based on an ammonia-to-hydrogen-to-power system. Green Chem. 2023, 25, 3888–3895.
- Otto, M.; Vesely, L.; Kapat, J.; Stoia, M.; Applegate, N.D.; Natsui, G. Ammonia as an Aircraft Fuel: A Critical Assessment From Airport to Wake. ASME Open J. Eng. 2023, 2, 021033.
- Chorowski, M.; Lepszy, M.; Machaj, K.; Malecha, Z.; Porwisiak, D.; Porwisiak, P.; Rogala, Z.; Stanclik, M. Challenges of Application of Green Ammonia as Fuel in Onshore Transportation. Energies 2023, 16, 4898.
- Takahashi, T.T. Maneuvering Capabilities of Hypersonic Airframes. In Proceedings of the AIAA SCITECH 2023 Forum, Online, 23–27 January 2023.
- Hewlett, S.G.; Pugh, D.G.; Valera-Medina, A.; Giles, A.; Runyon, J.; Goktepe, B.; Bowen, P.J. Industrial wastewater as an enabler of green ammonia to power via gas turbine technology. In Proceedings of the ASME Turbo Expo, Online, 21–25 September 2020.
- Hewlett, S.G.; Valera-Medina, A.; Pugh, D.G.; Bowen, P.J. Gas turbine co-firing of steelworks ammonia with coke oven gas or methane: A fundamental and cycle analysis. In Proceedings of the ASME Turbo Expo, Phoenix, AZ, USA, 17–21 June 2019.
- Giddey, S.; Badwal, S.P.S.; Munnings, C.; Dolan, M. Ammonia as a Renewable Energy Transportation Media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239.
- Valera-Medina, A.; Pugh, D.G.; Marsh, P.; Bulat, G.; Bowen, P. Preliminary study on lean premixed combustion of ammonia-hydrogen for swirling gas turbine combustors. Int. J. Hydrogen Energy 2017, 42, 24495–24503.
- Park, Y.-K.; Kim, B.-S. Catalytic removal of nitrogen oxides (NO, NO2, N2O) from ammonia-fueled combustion exhaust: A review of applicable technologies. Chem. Eng. J. 2023, 461, 141958.
- Kohse-Höinghaus, K. Combustion, Chemistry, and Carbon Neutrality. Chem. Rev. 2023, 123, 5139–5219.
- Shchepakina, E.A.; Zubrilin, I.A.; Kuznetsov, A.Y.; Tsapenkov, K.D.; Antonov, D.V.; Strizhak, P.A.; Yakushkin, D.V.; Ulitichev, A.G.; Dolinskiy, V.A.; Hernandez Morales, M. Physical and Chemical Features of Hydrogen Combustion and Their Influence on the Characteristics of Gas Turbine Combustion Chambers. Appl. Sci. 2023, 13, 3754.
- Mathieu, O.; Petersen, E.L. Carbon-Free Fuels; American Chemical Society: Washington, DC, USA, 2023.
- Shah, Z.A.; Mehdi, G.; Congedo, P.M.; Mazzeo, D.; De Giorgi, M.G. A review of recent studies and emerging trends in plasma-assisted combustion of ammonia as an effective hydrogen carrier. Int. J. Hydrogen Energy, 2023, in press.
- Zhai, L.; Liu, S.; Xiang, Z. Ammonia as a carbon-free hydrogen carrier for fuel cells: A perspective. Ind. Chem. Mater. 2023, 1, 332–342.
- Zhang, J.; Li, X.; Zheng, J.; Du, M.; Wu, X.; Song, J.; Cheng, C.; Li, T.; Yang, W. Non-thermal plasma-assisted ammonia production: A review. Energy Convers. Manag. 2023, 293, 117482.
- Aalrebei, O.F.; Al Assaf, A.H.; Amhamed, A.; Swaminathan, N.; Hewlett, S. Ammonia-hydrogen-air gas turbine cycle and control analyses. Int. J. Hydrogen Energy 2022, 47, 8603–8620.
- Bozo, M.G.; Vigueras-Zuniga, M.O.; Buffi, M.; Seljak, T.; Valera-Medina, A. Fuel rich ammonia-hydrogen injection for humidified gas turbines. Appl. Energy 2019, 251, 113334.
- Kang, L.; Pan, W.; Zhang, J.; Wang, W.; Tang, C. A review on ammonia blends combustion for industrial applications. Fuel 2023, 332, 126150.
- Kumuk, O.; Ilbas, M. Comparative analysis of ammonia/hydrogen fuel blends combustion in a high swirl gas turbine combustor with different cooling angles. Int. J. Hydrogen Energy, 2023, in press.
- Valera-Medina, A.; Goktepe, B.; Santhosh, R.; Runyon, J.; Giles, A.; Pugh, D.; Marsh, R.; Bowen, P. Ammonia gas turbines (AGT): Review. European Turbine Network (ETN) Proc. In Proceedings of the Future of Gas Turbine Technology 9th International Gas Turbine Conference, Brussels, Belgium, 10–11 October 2018.
- Kohansal, M.; Kiani, M.; Masoumi, S.; Nourinejad, S.; Ashjaee, M.; Houshfar, E. Experimental and Numerical Investigation of NH3/CH4 Mixture Combustion Properties under Elevated Initial Pressure and Temperature. Energy Fuels 2023, 37, 10681–10696.
- Xiao, H.; Valera-Medina, A. Chemical Kinetic Mechanism Study on Premixed Combustion of Ammonia/Hydrogen Fuels for Gas Turbine Use. J. Eng. Gas Turbines Power-Trans. Asme 2017, 139, 081504.
- Valera-Medina, A.; Morris, S.; Runyon, J.; Pugh, D.G.; Marsh, R.; Beasley, P.; Hughes, T. Ammonia, methane and hydrogen for gas turbines. Energy Procedia 2015, 75, 118–123.
- Lindfors, J. Performance of Cracked Ammonia Combustion in a Gas Turbine Engine-Evaluation through CFD and Chemical Reactor Network Modeling. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2022.
- Tyler, C. Ammonia as a source of hydrogen for hardening oils. Oil Soap 1934, 11, 231.
- Ikäheimo, J.; Kiviluoma, J.; Weiss, R.; Holttinen, H. Power-to-ammonia in future North European 100% renewable power and heat system. Int. J. Hydrogen Energy 2018, 43, 17295–17308.
- Alboshmina, N. Ammonia Cracking with Heat Transfer Improvement Technology; Cardiff University: Cardiff, UK, 2019.
- Lewis, B.; Von Elbe, G. Combustion, Flames and Explosions of Gases; Elsevier: Amsterdam, The Netherlands, 2012.
- Verkamp, F.J.; Hardin, M.C.; Williams, J.R. Ammonia combustion properties and performance in gas-turbine burners. Symp. (Int.) Combust. 1967, 11, 985–992.
- Brohi, E. Ammonia as Fuel for Internal Combustion Engines? Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2014.
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 269, 117448.
- Avery, W. A role for ammonia in the hydrogen economy. Int. J. Hydrogen Energy 1988, 13, 761–773.
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118.
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of Ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570.
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371.
- Xiao, H.; Valera-Medina, A.; Marsh, R.; Bowen, P.J. Numerical study assessing various ammonia/methane reaction models for use under gas turbine conditions. Fuel 2017, 196, 344–351.
- Pugh, D.; Bowen, P.; Valera-Medina, A.; Giles, A.; Runyon, J.; Marsh, R. Influence of steam addition and elevated ambient conditions on NOx reduction in a staged premixed swirling NH3/H2 flame. Proc. Combust. Inst. 2019, 37, 5401–5409.
- Mao, C.; Wang, P.; Wang, Y.; Valera Medina, A.; Cheng, K. Effects of equivalence ratio, inlet temperature and pressure on NO emissions for two stage combustion of NH3/H2 fuel mixture. In Proceedings of the 13th Asia-Pacific Conference on Combustion (ASPACC 2021), Abu Dhabi, United Arab Emirates, 5–9 December 2021.
- Shariff, S.W.M.; Sulaiman, S.; Azizul, M.A. Simulation and Modelling. The Spray Behaviour of Ammonia-Biodiesel Fuel Blends and Injector Characteristics in Micro-Gas Turbine. Prog. Eng. Appl. Technol. 2022, 3, 621–629.
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359.
- Iki, N.; Kurata, O.; Matsunuma, T.; Inoue, T.; Suzuki, M.; Tsujimura, T.; Furutani, H. Micro gas turbine firing kerosene and ammonia. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2015.
- Shmakov, A.; Korobeinichev, O.; Rybitskaya, I.; Chernov, A.; Knyazkov, D.; Bolshova, T.; Konnov, A. Formation and consumption of NO in H2 + O2 + N2 flames doped with NO or NH3 at atmospheric pressure. Combust. Flame 2010, 157, 556–565.
- Kumar, P.; Meyer, T.R. Experimental and modeling study of chemical-kinetics mechanisms for H2–NH3–air mixtures in laminar premixed jet flames. Fuel 2013, 108, 166–176.
- Powell, O.; Papas, P.; Dreyer, C. Flame structure measurements of NO in premixed hydrogen–nitrous oxide flames. Proc. Combust. Inst. 2011, 33, 1053–1062.
- Zhao, H.; Zhao, D.; Becker, S.; Zhang, Y. NO emission and enhanced thermal performances studies on Counter-flow Double-channel Hydrogen/Ammonia-fuelled microcombustors with Oval-shaped internal threads. Fuel 2023, 341, 127665.
- Lee, H.; Lee, M.J. Recent Advances in Ammonia Combustion Technology in Thermal Power Generation System for Carbon Emission Reduction. Energies 2021, 14, 5604.
- Somarathne, K.D.K.A.; Okafor, E.C.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Emission characteristics of turbulent non-premixed ammonia/air and methane/air swirl flames through a rich-lean combustor under various wall thermal boundary conditions at high pressure. Combust. Flame 2019, 210, 247–261.
- Hayakawa, A.; Arakawa, Y.; Mimoto, R.; Somarathne, K.D.K.A.; Kudo, T.; Kobayashi, H. Experimental investigation of stabilization and emission characteristics of ammonia/air premixed flames in a swirl combustor. Int. J. Hydrogen Energy 2017, 42, 14010–14018.
- Okafor, E.C.; Tsukamoto, M.; Hayakawa, A.; Somarathne, K.D.K.A.; Kudo, T.; Tsujimura, T.; Kobayashi, H. Influence of wall heat loss on the emission characteristics of premixed ammonia-air swirling flames interacting with the combustor wall. Proc. Combust. Inst. 2021, 38, 5139–5146.
- Pacheco, G.P.; Rocha, R.C.; Franco, M.C.; Mendes, M.A.A.; Fernandes, E.C.; Coelho, P.J.; Bai, X.S. Experimental and kinetic investigation of stoichiometric to rich NH3/H2/Air flames in a swirl and bluff-body stabilized burner. Energy Fuels 2021, 35, 7201–7216.
- Rocha, R.C.; Costa, M.; Bai, X.S. Combustion and Emission Characteristics of Ammonia under Conditions Relevant to Modern Gas Turbines. Combust. Sci. Technol. 2021, 193, 2514–2533.
- Kurata, O.; Iki, N.; Inoue, T.; Matsunuma, T.; Tsujimura, T.; Furutani, H.; Kawano, M.; Arai, K.; Okafor, E.C.; Hayakawa, A.; et al. Development of a wide range-operable, rich-lean low-NOx combustor for NH3 fuel gas-turbine power generation. Proc. Combust. Inst. 2019, 37, 4587–4595.
- Li, Z.X.; Li, S.H. Effects of inter-stage mixing on the NOx emission of staged ammonia combustion. Int. J. Hydrogen Energy 2022, 47, 9791–9799.
- Somarathne, K.D.K.A.; Okafor, E.C.; Sugawara, D.; Hayakawa, A.; Kobayashi, H. Effects of OH concentration and temperature on NO emission characteristics of turbulent non-premixed CH4/NH3/air flames in a two-stage gas turbine like combustor at high pressure. Proc. Combust. Inst. 2021, 38, 5163–5170.
- Somarathne, K.; Hatakeyama, S.; Hayakawa, A.; Kobayashi, H. Numerical study of a low emission gas turbine like combustor for turbulent ammonia/air premixed swirl flames with a secondary air injection at high pressure. Int. J. Hydrogen Energy 2017, 42, 27388–27399.
- Sun, Y.; Cai, T.; Shahsavari, M.; Sun, D.; Sun, X.; Zhao, D.; Wang, B. RANS simulations on combustion and emission characteristics of a premixed NH3/H2 swirling flame with reduced chemical kinetic model. Chin. J. Aeronaut. 2021, 34, 17–27.
- Duan, L.; Li, T. Research progress of ammonia combustion characteristic and stable-combustion technology. Huazhong Keji Daxue Xuebao (Ziran Kexue Ban)/J. Huazhong Univ. Sci. Technol. (Nat. Sci. Ed.) 2022, 50, 41–54.
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338.
- Lindstedt, R.; Lockwood, F.; Selim, M. Detailed kinetic modelling of chemistry and temperature effects on ammonia oxidation. Combust. Sci. Technol. 1994, 99, 253–276.
- Duynslaegher, C.; Contino, F.; Vandooren, J.; Jeanmart, H. Modeling of ammonia combustion at low pressure. Combust. Flame 2012, 159, 2799–2805.
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combust. Flame 2009, 156, 1413–1426.
- Li, J.; Huang, H.; Kobayashi, N.; Wang, C.; Yuan, H. Numerical study on laminar burning velocity and ignition delay time of ammonia flame with hydrogen addition. Energy 2017, 126, 796–809.
- Dagaut, P.; Nicolle, A. Experimental and kinetic modeling study of the effect of SO2 on the reduction of NO by ammonia. Proc. Combust. Inst. 2005, 30, 1211–1218.
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.-J.; Herzler, J.; Naumann, C.; Griebel, P.; Mathieu, O. An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures. Combust. Flame 2013, 160, 995–1011.
- Xiao, H.; Chen, A.; Zhang, M.; Guo, Y.; Ying, W. Using Ammonia as Future Energy: Modelling of Reaction Mechanism for Ammonia/Hydrogen Blends. J. Phys. Conf. Ser. 2022, 2361, 012012.
- Cai, T.; Zhao, D.; Gutmark, E. Overview of fundamental kinetic mechanisms and emission mitigation in ammonia combustion. Chem. Eng. J. 2023, 458, 141391.
- Wang, Z.; Yu, Z.; Chen, C.; He, Y.; Zhu, Y. Research progress on combustion characteristics of new zero carbon ammonia fuel. J. Huazhong Univ. Sci. Technol. (Nat. Sci. Ed.) 2022, 50, 27–40+78.
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4-NH3-air premixed flames. Combust. Flame 2018, 187, 185–198.
- Fenimore, C.; Jones, G. Oxidation of ammonia in flames. J. Phys. Chem. 1961, 65, 298–303.
- Miller, J.A.; Smooke, M.D.; Green, R.M.; Kee, R.J. Kinetic modeling of the oxidation of ammonia in flames. Combust. Sci. Technol. 1983, 34, 149–176.
- Skreiberg, Ø.; Kilpinen, P.; Glarborg, P. Ammonia chemistry below 1400 K under fuel-rich conditions in a flow reactor. Combust. Flame 2004, 136, 501–518.
- Han, X.; Wang, Z.; He, Y.; Zhu, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/syngas/air premixed flames. Combust. Flame 2020, 213, 1–13.
- Alnasif, A.; Mashruk, S.; Shi, H.; Alnajideen, M.; Wang, P.; Pugh, D.; Valera-Medina, A. Evolution of ammonia reaction mechanisms and modeling parameters: A review. Appl. Energy Combust. Sci. 2023, 15, 100175.
- Nakamura, H.; Hasegawa, S.; Tezuka, T. Kinetic modeling of ammonia/air weak flames in a micro flow reactor with a controlled temperature profile. Combust. Flame 2017, 185, 16–27.
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68.
- Wang, S.; Wang, Z.; Elbaz, A.M.; Han, X.; He, Y.; Costa, M.; Konnov, A.A.; Roberts, W.L. Experimental study and kinetic analysis of the laminar burning velocity of NH3/syngas/air, NH3/CO/air and NH3/H2/air premixed flames at elevated pressures. Combust. Flame 2020, 221, 270–287.
- Valera-Medina, A.; Banares-Alcantara, R. Techno-Economic Challenges of Green Ammonia as an Energy Vector; Academic Press: Cambridge, MA, USA, 2020.
- Hussein, N.A.; Valera-Medina, A.; Alsaegh, A.S. Ammonia-hydrogen combustion in a swirl burner with reduction of NOx emissions. Energy Procedia 2019, 158, 2305–2310.
- Mashruk, S.; Kovaleva, M.; Tung Chong, C.; Hayakawa, A.; Okafor, E.C.; Valera-Medina, A. Nitrogen oxides as a by-product of ammonia/hydrogen combustion regimes. Chem. Eng. Trans. 2021, 89, 613–618.
- Ammonia-Powered Gas Turbines. Available online: https://www.ge.com/gas-power/future-of-energy/ammonia-powered-gas-turbines (accessed on 10 August 2023).
- Wu, B. Keppel Breaks Ground for Singapore’s First Hydrogen Ready Cogeneration Plant. Company in the News 2023. Available online: https://www.theedgesingapore.com/news/company-news/keppel-breaks-ground-singapores-first-hydrogen-ready-cogeneration-plant (accessed on 10 August 2023).
- Bailey, M. Johnson Matthey and Doosan Enerbility Jointly Developing Integrated Ammonia-Cracking Projects. Sustainabilit 2023. Available online: https://www.chemengonline.com/johnson-matthey-and-doosan-enerbility-jointly-developing-integrated-ammonia-cracking-projects/ (accessed on 10 August 2023).
- Young-sil, Y. Korea Expediting Creation of World’s First Hydrogen Power Tender Market. Power Generation Avenues 2023. Available online: http://www.businesskorea.co.kr/news/articleView.html?idxno=120138 (accessed on 10 August 2023).
- Park, H.-S. Hanwha Impact Develops 50% Hydrogen Gas Turbine Technology. Energy 2023. Available online: https://www.kedglobal.com/energy/newsView/ked202306220011 (accessed on 10 August 2023).
- Čučuk, A. MHI Launches Operations at Nagasaki Carbon Neutral Park. Business Developments & Projects 2023. Available online: https://www.offshore-energy.biz/mhi-launches-operations-at-nagasaki-carbon-neutral-park/ (accessed on 10 August 2023).
- Smith, M. Australia Fuels Japan’s Big Bet on Ammonia. North Asia Correspondent 2023. Available online: https://www.afr.com/world/asia/australia-fuels-japan-s-big-bet-on-ammonia-20230709-p5dmux (accessed on 10 August 2023).
- Otto, M.; Chagoya, K.L.; Blair, R.G.; Hick, S.M.; Kapat, J.S. Optimal hydrogen carrier: Holistic evaluation of hydrogen storage and transportation concepts for power generation, aviation, and transportation. J. Energy Storage 2022, 55, 105714.
- Kerrebrock, J.L. Aircraft Engines and Gas Turbines; MIT Press: Cambridge, MA, USA, 1992.
- Langston, L. The Adaptable Gas Turbine. 2013. Available online: https://www.americanscientist.org/article/the-adaptable-gas-turbine (accessed on 19 March 2023).
- Poullikkas, A. An overview of current and future sustainable gas turbine technologies. Renew. Sustain. Energy Rev. 2005, 9, 409–443.
- Yee, S.K.; Milanovic, J.V.; Hughes, F.M. Overview and comparative analysis of gas turbine models for system stability studies. IEEE Trans. Power Syst. 2008, 23, 108–118.
- Thitakamol, B.; Veawab, A.; Aroonwilas, A. Environmental impacts of absorption-based CO2 capture unit for post-combustion treatment of flue gas from coal-fired power plant. Int. J. Greenh. Gas Control 2007, 1, 318–342.
- Singer, R. New materials for industrial gas turbines. Mater. Sci. Technol. 1987, 3, 726–732.
- Glenny, R.; Northwood, J.; Smith, A.B. Materials for gas turbines. Int. Metall. Rev. 1975, 20, 1–28.
- Schilke, P.; Foster, A.; Pepe, J. Advanced Gas Turbine Materials and Coatings; General Electric Company: New York, NY, USA, 1991.
- Singh, K. Advanced materials for land based gas turbines. Trans. Indian Inst. Met. 2014, 67, 601–615.
- Biswas, S.; Ramachandra, S.; Hans, P.; Kumar, S.S. Materials for Gas Turbine Engines: Present Status, Future Trends and Indigenous Efforts. J. Indian Inst. Sci. 2022, 102, 297–309.
- Van Eck, N.J.; Waltman, L. VOSviewer Manual. Man; VOSviewer Version 1.6.15; Centre for Science and Technology Studies, Leiden University: Leiden, The Netherlands, 2015; Available online: https://www.vosviewer.com (accessed on 1 April 2020).
- Bakan, E.; Mack, D.E.; Mauer, G.; Vaßen, R.; Lamon, J.; Padture, N.P. High-temperature materials for power generation in gas turbines. In Advanced Ceramics for Energy Conversion and Storage; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–62.
- Stefan, E.; Talic, B.; Larring, Y.; Gruber, A.; Peters, T.A. Materials challenges in hydrogen-fuelled gas turbines. Int. Mater. Rev. 2022, 67, 461–486.
- Nathan, S. Jewel in the Crown: Rolls-Royce’s Single-Crystal Turbine Blade Casting Foundry. The Engineer Online. Available online: https://go.gale.com/ps/i.do?id=GALE%7CA417139592&sid=sitemap&v=2.1&it=r&p=AONE&sw=w&userGroupName=anon%7E37650ece&aty=open-web-entry2015 (accessed on 20 August 2023).
- Valera-Medina, A.; Gutesa, M.; Xiao, H.; Pugh, D.; Giles, A.; Goktepe, B.; Marsh, R.; Bowen, P. Premixed ammonia/hydrogen swirl combustion under rich fuel conditions for gas turbines operation. Int. J. Hydrogen Energy 2019, 44, 8615–8626.
- Zamfirescu, C.; Dincer, I. Using ammonia as a sustainable fuel. J. Power Sources 2008, 185, 459–465.
- Cornelius, W.; Huellmantel, L.W.; Mitchell, H.R. Ammonia as an engine fuel. SAE Trans. 1966, 300–326. Available online: https://www.sae.org/publications/technical-papers/content/650052/ (accessed on 20 August 2023).
- Westlye, F.R.; Ivarsson, A.; Schramm, J. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel 2013, 111, 239–247.
- Xu, X.; Liu, E.; Zhu, N.; Liu, F.; Qian, F. Review of the Current Status of Ammonia-Blended Hydrogen Fuel Engine Development. Energies 2022, 15, 1023.
- Lee, J.; Kim, J.; Park, J.; Kwon, O. Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 1054–1064.
- Božo, M.G.; Mashruk, S.; Zitouni, S.; Valera-Medina, A. Humidified ammonia/hydrogen RQL combustion in a trigeneration gas turbine cycle. Energy Convers. Manag. 2021, 227, 113625.
- Lefebvre, A.H.; Ballal, D.R. Gas Turbine Combustion: Alternative Fuels and Emissions; CRC Press: Boca Raton, FL, USA, 2010.
- Feitelberg, A.S.; Jackson, M.R.; Lacey, M.A.; Manning, K.S.; Ritter, A.M. Design and Performance of a Low Btu Fuel Rich-Quench-Lean Gas Turbine Combustor; USDOE Morgantown Energy Technology Center (METC): Morgantown, WV, USA, 1996.
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416.
- De Paepe, W.; Carrero, M.M.; Bram, S.; Contino, F.; Parente, A. Waste heat recovery optimization in micro gas turbine applications using advanced humidified gas turbine cycle concepts. Appl. Energy 2017, 207, 218–229.
- Huang, Z.; Yang, C.; Yang, H.; Ma, X. Off-design heating/power flexibility for steam injected gas turbine based CCHP considering variable geometry operation. Energy 2018, 165, 1048–1060.
- Zhang, C.; Wang, X.; Yang, C.; Yang, Z. Control strategies of steam-injected gas turbine in CCHP system. Energy Procedia 2017, 105, 1520–1525.
- Larson, E.D.; Williams, R.H. Steam-injected gas turbines. J. Eng. Gas Turbines Power 1987, 109, 55–63.
- Jones, J.; Flynn, B.; Strother, J. Operating Flexibility and Economic Benefits of a Dual-Fluid Cycle 501-KB Gas Turbine Engine in Cogeneration Applications. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 1982.
- Huzel, D.K.; Huang, D.H. Modern engineering for design of liquid-propellant rocket engines (Revised and enlarged edition). In Progress in Astronautics and Aeronautics; American Institute of Aeronautics & Astronautics: Reston, VA, USA, 1992; Volume 147.
- Pollock, T.M. Alloy design for aircraft engines. Nat. Mater. 2016, 15, 809–815.
- Lee, J.A.; Woods, S. Hydrogen Embrittlement; NASA: Washington, DC, USA, 2016.
- González, M.S.; Rojas-Hernández, I. Hydrogen embrittlement of metals and alloys in combustion engines. Tecnol. Marcha 2018, 31, 3–13.
- Alhuyi Nazari, M.; Fahim Alavi, M.; Salem, M.; Assad, M.E.H. Utilization of hydrogen in gas turbines: A comprehensive review. Int. J. Low-Carbon Technol. 2022, 17, 513–519.
- Stuen, T.H. Influence of Hydrogen Use As a Fuel on Aeroderivative Gas Turbine Performance; NTNU: Trondheim, Norway, 2021.
- Duarte, M.J.; Fang, X.; Rao, J.; Krieger, W.; Brinckmann, S.; Dehm, G. In situ nanoindentation during electrochemical hydrogen charging: A comparison between front-side and a novel back-side charging approach. J. Mater. Sci. 2021, 56, 8732–8744.
- Lagow, B.W. Materials selection in gas turbine engine design and the role of low thermal expansion materials. JOM 2016, 68, 2770–2775.
- Kıymaz, T.B.; Böncü, E.; Güleryüz, D.; Karaca, M.; Yılmaz, B.; Allouis, C.; Gökalp, İ. Numerical investigations on flashback dynamics of premixed methane-hydrogen-air laminar flames. Int. J. Hydrogen Energy 2022, 47, 25022–25033.
- Davies, M. Corrosion by Ammonia. ASM Handb. 2006, 13, 727–735. Available online: https://www.osti.gov/biblio/6371012 (accessed on 20 August 2023).
- Loginow, A. A review of stress corrosion cracking of steel in liquefied ammonia service. Mater. Perform. 1986, 25, 8–22.
- Davalos-Monteiro, R. Observations of corrosion product formation and stress corrosion cracking on brass samples exposed to ammonia environments. Mater. Res. 2018, 22, e20180077.
- Bordenet, B.; Singheiser, L. High Temperature Corrosion in Gas Turbines: Thermodynamic Modelling and Experimental Results; Fakultät für Maschinenwesen: Aachen, Germany, 2004.
- Wu, W.; Wei, B.; Li, G.; Chen, L.; Wang, J.; Ma, J. Study on ammonia gas high temperature corrosion coupled erosion wear characteristics of circulating fluidized bed boiler. Eng. Fail. Anal. 2022, 132, 105896.
- Fathyunes, L.; Mohtadi-Bonab, M. A Review on the Corrosion and Fatigue Failure of Gas Turbines. Metals 2023, 13, 701.
- Ma, D. Novel casting processes for single-crystal turbine blades of superalloys. Front. Mech. Eng. 2018, 13, 3–16.
- Wee, S.; Do, J.; Kim, K.; Lee, C.; Seok, C.; Choi, B.-G.; Choi, Y.; Kim, W. Review on mechanical thermal properties of superalloys and thermal barrier coating used in gas turbines. Appl. Sci. 2020, 10, 5476.
- Smialek, J.L.; Miller, R.A. Revisiting the birth of 7YSZ thermal barrier coatings: Stephan Stecura. Coatings 2018, 8, 255.
- Han, J.-C. Fundamental gas turbine heat transfer. J. Therm. Sci. Eng. Appl. 2013, 5, 021007.
- Gok, M.G.; Goller, G. State of the art of gadolinium zirconate based thermal barrier coatings: Design, processing and characterization. In Methods for Film Synthesis and Coating Procedures; IntechOpen: London, UK, 2019.
- Boyce, M.P. Gas Turbine Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2011.
- Alexeev, R.; Tishchenko, V.; Gribin, V.; Gavrilov, I.Y. Turbine blade profile design method based on Bezier curves. J. Phys. Conf. Ser. 2017, 891, 012254.
- Gribin, V.; Tishchenko, V.; Alexeev, R. Turbine blade profile design using bezier curves. In Proceedings of the 12th European Conference on Turbomachinery Fluid Dynamics and Thermodynamics, ETC 2017, Stockholm, Sweden, 3–7 April 2017.
- Blazek, J. Computational fluid dynamics: Principles and applications; Butterworth-Heinemann: Oxford, UK, 2015.
- Singh, M.P.; PE, G.M.L. Blade Design and Analysis for Steam Turbines; McGraw-Hill Education: New York, NY, USA, 2011.
- Halls, G. Air Cooling of Turbine Blades and Vanes: An account of the history and development of gas turbine cooling. Aircr. Eng. Aerosp. Technol. 1967, 39, 4–14.
- Wright, L.M.; Han, J.-C. Enhanced internal cooling of turbine blades and vanes. Gas Turbine Handb. 2006, 4, 1–5.
- Han, J.-C.; Rallabandi, A. Turbine blade film cooling using PSP technique. Front. Heat Mass Transf. 2010, 1, 013001.
- Sanz, W. Design of Thermal Turbomachinery; Ankara, Institute for Thermal Turbomachinery and Machine Dynamics, Graz University of Technology: Graz, Austria, 2008.
- Soares, C. Gas turbines in simple cycle & combined cycle applications. In The Gas Turbine Handbook; NETL: Morgantown, WV, USA, 1998.
- Latcovich, J.A., Jr.; Bach, C.S. Field Evaluation and Operating Experience of the Allison 501-KB5 Industrial Gas Turbine. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 1986.
- Bloch, H.P.; Soares, C. Process Plant Machinery; Elsevier: Amsterdam, The Netherlands, 1998.
- Royce, R. Rolls Royce; Rolls Royce: Derby, UK, 2018.
- Alkebsi, E.A.A.; Ameddah, H.; Outtas, T.; Almutawakel, A. Design of graded lattice structures in turbine blades using topology optimization. Int. J. Comput. Integr. Manuf. 2021, 34, 370–384.
- Yvon, P.; Carré, F. Structural materials challenges for advanced reactor systems. J. Nucl. Mater. 2009, 385, 217–222.
- Walter, K.; Greaves, W. Life assessment of gas turbine components using nondestructive inspection techniques. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 1997.
- Ramakrishnan, A.; Dinda, G. Direct laser metal deposition of Inconel 738. Mater. Sci. Eng. A 2019, 740, 1–13.
- Fanijo, E.O.; Thomas, J.G.; Zhu, Y.; Cai, W.; Brand, A.S. Surface Characterization Techniques: A Systematic Review of their Principles, Applications, and Perspectives in Corrosion Studies. J. Electrochem. Soc. 2022. Available online: https://iopscience.iop.org/article/10.1149/1945-7111/ac9b9b/meta (accessed on 20 August 2023).
- Bogdan, M.; Błachnio, J.; Kułaszka, A.; Zasada, D. Investigation of the Relationship between Degradation of the Coating of Gas Turbine Blades and Its Surface Color. Materials 2021, 14, 7843.
- Livings, R.; Smith, N.; Biedermann, E.; Scheibel, J. Process Compensated Resonance Testing for Qualifying the Metallurgical Aspects and Manufacturing Defects of Turbine Blades. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2020.
- Karaoglanli, A.C.; Ogawa, K.; Turk, A.; Ozdemir, I. Thermal shock and cycling behavior of thermal barrier coatings (TBCs) used in gas turbines. Prog. Gas Turbine Perform. 2013, 2013, 237–260.
- Laguna-Camacho, J.; Villagrán-Villegas, L.; Martínez-García, H.; Juárez-Morales, G.; Cruz-Orduña, M.; Vite-Torres, M.; Ríos-Velasco, L.; Hernández-Romero, I. A study of the wear damage on gas turbine blades. Eng. Fail. Anal. 2016, 61, 88–99.
- Gallardo, J.; Rodríguez, J.; Herrera, E. Failure of gas turbine blades. Wear 2002, 252, 264–268.
- Choudhary, O.; Kalita, P.; Doley, P.; Kalita, A. 1. Scanning Electron Microscope-Advantages and Disadvantages in Imaging Components by OP Choudhary, PC Kalita, PJ Doley and A. Kalita. Life Sci. Leafl. 2017, 85, 1–7.
- Hodoroaba, V.-D. Energy-dispersive X-ray spectroscopy (EDS). In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 397–417.
- Teixeira, Ó.; Silva, F.J.; Atzeni, E. Residual stresses and heat treatments of Inconel 718 parts manufactured via metal laser beam powder bed fusion: An overview. Int. J. Adv. Manuf. Technol. 2021, 113, 3139–3162.
- Sun, F.; Tong, J.; Feng, Q.; Zhang, J. Microstructural evolution and deformation features in gas turbine blades operated in-service. J. Alloys Compd. 2015, 618, 728–733.
- Kalita, O.C.P.; Doley, P.; Kalita, A. 2. Uses of Transmission Electron Microscope in Microscopy and Its Advantages and Disadvantages by OP Choudhary, PC Kalita, PJ Doley and A. Kalita. Life Sci. Leafl. 2017, 85, 8–13.
- Gadegaard, N. Atomic force microscopy in biology: Technology and techniques. Biotech. Histochem. 2006, 81, 87–97.
- Shinato, K.W.; Huang, F.; Jin, Y. Principle and application of atomic force microscopy (AFM) for nanoscale investigation of metal corrosion. Corros. Rev. 2020, 38, 423–432.
- Lee, D.W.; Cho, S.S.; Hong, S.H.; Joo, W.S. Failure analysis of turbine blade in atomic power plant. J. Mech. Sci. Technol. 2008, 22, 864–870.
- Kovalev, A.; Tishchenko, L.; Shashurin, V.; Galinovskii, A. Application of X-ray diffraction methods to studying materials. Russ. Metall. (Met.) 2017, 2017, 1186–1193.
- Nakai, I.; Abe, Y. Portable X-ray powder diffractometer for the analysis of art and archaeological materials. Appl. Phys. A 2012, 106, 279–293.
- Wilson, T. Scanning optical microscopy. Scanning 1985, 7, 79–87.
- Kiran Attota, R. Through-focus scanning optical microscopy applications. In Unconventional Optical Imaging; SPIE: Gaithersburg, MD, USA, 2018.
- Nellist, P.D. Scanning transmission electron microscopy. In Springer Handbook of Microscopy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 49–99.
- Τawancy, H.; Al-Hadhrami, L.M. Degradation of turbine blades and vanes by overheating in a power station. Eng. Fail. Anal. 2009, 16, 273–280.
- Hovis, D.; Heuer, A. The use of laser scanning confocal microscopy (LSCM) in materials science. J. Microsc. 2010, 240, 173–180.
- Paddock, S.W. Principles and practices of laser scanning confocal microscopy. Mol. Biotechnol. 2000, 16, 127–149.
- Jiao, J.; Xu, Z.; Zan, S.; Zhang, W.; Sheng, L. Research on microstructure properties of the TiC/Ni-Fe-Al coating prepared by laser cladding technology. In Proceedings of the AOPC 2017: Laser Components, Systems, and Applications, Beijing, China, 4–6 June 2017.
More