Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shuhua Zhu and Version 2 by Camila Xu.
Hydrogen sulfide (H2S), as an environmental toxin, is now confirmed to be a biological mediator and plays essential roles in normal physiology and in the responses to different stresses. H2S also regulates the responses to oxidative stress by interplaying with reactive oxygen species (ROS) at multiple levels and protects mitochondrial function, maintaining mitochondrial homeostasis.
- hydrogen sulfide
- mitochondria
- oxidative phosphorylation
- tricarboxylic acid cycle
1. Introduction
Hydrogen sulfide (H2S), as an environmental toxin, is now confirmed to be a biological mediator and plays essential roles in normal physiology and in the responses to different stresses [1][2][1,2]. H2S also regulates the responses to oxidative stress by interplaying with reactive oxygen species (ROS) at multiple levels [3][4][3,4] and protects mitochondrial function [5][6][5,6], maintaining mitochondrial homeostasis [7]. Mitochondria are the cells’ oxidation centers and power stations; they coordinate cell metabolism and immunity [8], and are both the source and the target of H2S. H2S can be produced inside or outside mitochondria, regulating mitochondrial energy metabolism, and maintaining mitochondrial functions under stress [3][9][3,9]. In mitochondria, the tricarboxylic acid (TCA) cycle is the final metabolic pathway of the three major nutrients (sugars, lipids, and amino acids) and the hub of the metabolism of sugars, lipids, and amino acids. The TCA cycle is a step in the process of respiration, after which high energy electrons are oxidized and ADPs are phosphorylated through the electron transport chain with the help of NADH, H+, and FADH2 to produce ATPs [10]. The mitochondrial respiratory chain, also called an electron transfer chain, is a continuous reaction system composed of a series of hydrogen transfer reactions and electron transfer reactions arranged in a specific order; it produces the majority of ROS, and supplies the cell with energy [11]. Respiratory chain complex I (NADH-ubiquinone oxidoreductase) oxidizes NADH, pumps protons from the inside of the mitochondrial inner membrane to the membrane gap, and transfers electrons to ubiquinone; complex II (succinate dehydrogenase) has a role in transferring electrons from succinic acid to ubiquinone; complex III (ubiquinone-cytochrome c oxidoreductase), an essential mitochondrial protein complex in the oxidative phosphorylation process, transfers electrons from ubiquinone to cytochrome c; complex IV (cytochrome c oxidase) pumps protons into the membrane gap and transfers electrons from cytochrome c to oxygen. These protons drive ATP synthesis by ATP synthase [12]. Disorder in the mitochondrial respiratory complexes is an essential cause of mitochondrial disease and aging [13].
2. Chemical and Biological Characteristics of H2S
H2S, as a colorless, corrosive gas, is poisonous and even lethal at high concentrations [14]; as a lipophilic molecule, it can diffuse readily through biological membranes. As a polar and hydrogen-bonding-capable molecule, H2S has a membrane permeability comparable to O2 and CO2, which are nonpolar. H2S can cross lipid bilayers with permeability coefficients from 0.5 cm/s to about 12 cm/s, which depend on the different membranes [15]. The solubility of H2S in pure water is up to 3.846 mg/g at 20 °C, and aqueous H2S is volatile due to its dissociation [16]. More than 80% of H2S in the water at physiological pH is dissociated to hydrosulfide anion (HS−) and then dissociated to sulfide anion (S2−) at a higher pH, and the rest of the H2S remains as an undissociated molecule [17]. H2S, as a weak diprotic acid, has pKa1 values of 6.98 at 25 °C and 6.76 at 37 °C [14]. Therefore, the availability of HS− is high at neutral pH in vivo. The pKa values of the second dissociation are 17~19 at 25 °C [14]. Thus, alkaline sodium sulfide (Na2S) and sodium hydrosulfide (NaHS) solution can be applied as H2S sources to lower the pH.
The energies of orbitals for H2S (−10.47 eV) are lower than those of HS− (−2.31 eV), indicating that the nucleophilicity of HS− is higher than that of H2S [17]. With its negative charge and low electronegativity, HS− can form a covalent bond with an electrophile (E+) by donating a pair of electrons, producing E-SH, and the product E-SH can also react with another E+ to form E-S-E [14]. This reactivity is the basis of the biological effect of H2S.
The chemical properties of H2S (or HS−) as nucleophiles give the possibilities for two kinds of interaction between H2S and metals (Figure 1) [18]: (i) H2S (HS−) can bind noncovalently or coordinate the transitional metals as a ligand; (ii) H2S (HS−) can reduce the metal, accompanied by the production of HS• and other downstream sulfur oxidation products. The positively charged transitional metal ions, such as iron and copper ions, can change valence by accepting an electron.
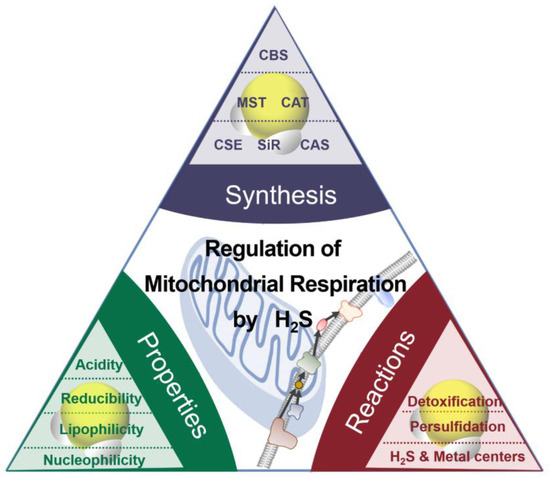
Figure 1.
The synthesis, properties, and reaction of H
2
S.
Cytochrome c oxidase (CcO), as a mitochondrial hemeprotein, contains copper centers, which are CuA and CuB, and ferric heme a and a3 [19]. H2S can bind to and reduce CcO and serve as an electron donor. Ferric heme a3 can oxidize H2S with low concentrations to be HS•. The product HS• is likely to react with HS− to produce H2S2•−. Alternatively, HS• can be oxidized by oxygen to form HSOO•. Despite inhibiting CcO, heme iron reduction promotes oxygen consumption, resulting in the stimulation of respiration [14][20][14,20]. At high levels, H2S binds to the O2-binding CuB center to be a Cu-SH complex that cannot be re-oxidized. Excessive H2S coordinates to ferric heme a3, forming a Fe-SH complex, eventually leading to irreversible inhibition of CcO. Cytochrome c has a similar behavior in that heme ferric iron is reduced by H2S. Therefore, more reducing agents enter the electron transfer chain, consuming more oxygen. The inhibition of cytochrome c by H2S, to some extent, promotes CcO reduction and respiration [21].
H2S, a covalent hydride, is considered the simplest thiol, and its bond dissociation energy is about 385 kJ/mol, which is similar to that of the S-H bond in other thiols [22]. Both H2S and HS− act as reductants. H2S can be oxidized by oxidants to substances with higher oxidation states, including sulfur (S0), sulfur dioxide (SO2), sulfite (SO32−), sulfate (SO42−), sulfur trioxide (SO3), and thiosulfate (S2O3−), and sulfonyl radical (HS•).
H2S can be oxidized by several biologically reactive species, such as nitrogen dioxide, hydroxyl radicals, peroxyl radicals, and superoxide radicals. The HS• is the initial oxidation product of H2S. HS• can be transformed into SO2•− under the oxidation of O2, while O2 is catalyzed to be a superoxide radical (O2•−). O2•− can be dismutated by superoxide dismutase into O2 and H2O2. The nucleophilic substitution of HS− on H2O2 forms polysulfanes. Sulfenic acid (HSOH) formed by the reaction between HS− and hydroperoxides (ROOH) can be transformed into HSSH by reacting with another HS−. The nucleophilic attack of HS− on peroxynitrite (ONOOH) gives HSOH and NO2−. •NO is involved in many vital physiological processes and signaling in mammals and plants and has complex crosstalk with H2S signaling. H2S can reduce •NO to form nitroxyl (HNO) or nitrososulfane (HSNO•−), eventually leading to N2O and sulfane sulfur formation [23]. Oxidization of •NO to NO2− can also be facilitated by H2S. H2S can stimulate the formation of S-nitrosothiols (RSNO) of cysteine caused by •NO.
In addition to S-nitrosothiols, persulfide (RSSH/RSS−) can be formed from the posttranslational modification of cysteines by H2S (HS−). H2S also reacts with GSSG to generate glutathione persulfide (GSSH) [24]. H2S can transfer sulfur with the catalysis of sulfide quinone oxidoreductase (SQR) to GSH to form GSSH [25].
Apart from their similar characteristics to thiols, disulfides, polysulfides, and hydroperoxides, persulfides attract increasing attention in biology as versatile molecules. Compared with thiols and H2S, persulfides are predicted to be more acidic and nucleophilic with a weaker S-H bond whose dissociation energy is 293 kJ/mol [26]. Thus, RSSH can reduce ferric cytochrome c to ferrous cytochrome c with the concomitant generation of RSS•. The cysteine residues modify the sulfur transferase (ST) structures involved in the H2S-producing process. The sulfur of the active site of the protein persulfides can be catalyzed by these enzymes to be thiols or sulfite. H2S can react with protein sulfenic acids (RSOH) to form persulfides (RSSO2H/RSSO3H) [14]. Iron-sulfur (Fe-S) clusters, as inorganic cofactors, especially bind to respiratory complexes, becoming involved in fundamental life processes such as energy production as well as electron transfer. The generation of persulfides (RSSH) involves Fe-S cluster synthesis, which is the crucial step of Fe-S assembly in mitochondria [27]. Persulfides are unstable in solution at room temperature and react with the outer and inner sulfur, yielding sulfur and H2S (HS−) [28]. Persulfides/polysulfides contain sulfane sulfur, which has six valence electrons and no charge [29] and is mainly responsible for the biological activity attributed to H2S [30]. H2S is synthesized by the same enzymes involved in forming sulfane sulfur [31], suggesting a close relationship between H2S and sulfane sulfur and that these two reactive sulfur species always coexist [32][33][32,33]. It has been suggested that it is rather a sulfane sulfur, and not the H2S itself, that acts as a signaling molecule and is responsible for the biological actions of RSS. The term H2S is still used for narrative convenience in the following text.
Thus, H2S can signal through reduction and/or direct binding of metalloprotein heme centers, potent antioxidants through reactive oxygen species/reactive nitrogen species scavenging, and modifying proteins through persulfidation [5].
