1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a condition that includes a broad spectrum of histological abnormalities, including isolated steatosis, non-alcoholic steatohepatitis (NASH), and liver fibrosis, which can eventually lead to cirrhosis, liver cancer, and death. NAFLD affects 20–25% of the adult population, and it is estimated that 20% develop steatohepatitis
[1].
The pathogenesis of non-alcoholic fatty liver disease (NAFLD) is multifactorial. The multiple-hit hypothesis implicates several factors as causes of this disease. The most notable factors are genetics, obesity, sedentary lifestyle, high-fat diet, insulin resistance, and gut microbiota
[2]. According to this theory, there is a vicious circle of fat accumulation in hepatocytes, lipotoxicity, metabolic disorders, inflammation, insulin resistance, and aggravation of metabolic disorders
[3].
In addition to the well-known risk factors for the disease, interactions between the gut microbiome, its derived metabolites, the immune system, and the liver contribute to the pathogenesis of NAFLD
[4].
2. Gut–Liver Axis
Gut microbiota refers to a complex community of microorganisms found in the digestive tract of humans and animals
[5]. The gut microbiota is considered a virtual metabolic organ that forms an axis with various extraintestinal organs (kidneys, brain, cardiovascular system, etc.); however, in recent years, the gut–liver axis has attracted the attention of researchers
[6]. The gut–liver axis is the anatomical and functional bidirectional relationship between the gut and the liver
[4][6][4,6].
The portal vein is an anatomical reference point for gut–liver communication and establishes the interaction between the intestinal microbiome and the liver, as it is responsible for transporting gut-derived products to the hepatic circulation
[4][7][4,7] (
Figure 1A). This explains why alterations in the intestinal barrier can lead to the entry of pathogens or their products into the liver, where they can cause or worsen liver diseases
[4].
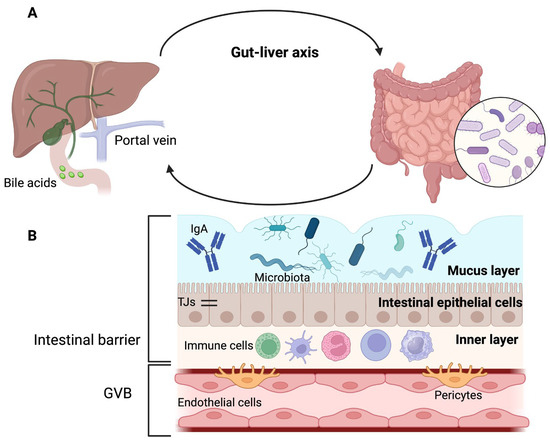
Figure 1. Gut–liver axis and intestinal barrier. (
A) Gut, portal circulation, liver, and bile duct are connected anatomically. (
B) The intestinal barrier has three layers: the outer layer is made up of mucus, microbiota, and defense proteins such as immunoglobulin A (IgA); the middle layer corresponds to intestinal epithelial cells that are sealed together by tight junctions (TJs), and the inner layer is composed of immune cells. The gut–vascular barrier (GVB) constitutes a second protective barrier. Adapted from ref.
[4]. Created by BioRender (accessed on 29 August 2023).
The intestinal barrier (
Figure 1B) has an outer layer of mucus, microbiota, and defense proteins such as secretory immunoglobulin A and antimicrobial proteins. The middle layer of the intestinal barrier corresponds to the intestinal epithelial cells, and the inner layer comprises immune cells
[8].
The mucus layer is mainly water and contains glycoproteins known as mucins (MUC), produced by goblet cells
[8][9][10][8,9,10]. The outer layer of mucus is colonized by microbiota, whereas the inner layer is sterile
[4]. The stomach and colon contain both mucus layers; however, only one layer is present in the small intestine. Therefore, the mucus layer of the small intestine is more penetrable by bacteria and toxins. To compensate for the absence of one of these layers in the small intestine, enterocytes, Paneth cells, or immune cells secrete antimicrobial proteins
[8][10][8,10].
Below the mucus layer, epithelial cells are sealed together by tight junctions (TJs) that release antimicrobial peptides for host defense
[4][11][4,11]. In addition, the brush border of enterocytes is negatively charged and opposes the negative charge of the microbiota
[4].
TJs have two pathways that allow the passage of substances; the first is the “pore” pathway, which is highly selective, and the second is the “leak” pathway, which shows limited selectivity
[8].
It has been proposed that in addition to the intestinal epithelial barrier, there is a second intestinal barrier called the gut–vascular barrier (GVB). The GVB comprises of endothelial cells, enteric glial cells, and pericytes that prevent the entry of intestinal microorganisms into the body
[11][12][11,12]. However, certain bacteria have developed mechanisms to overcome this barrier
[4].
In addition to portal circulation, the gut and liver communicate through the flow of hepatic bile
[9]. Bile acids are synthesized in the liver and released into the gut, where microbiota further metabolizes them. Microbiota and bile acids have a bidirectional interaction; bile acids affect the composition of the microbiota, which in turn affects bile acid metabolism
[4][7][13][4,7,13]. Furthermore, altered bile acid metabolism may promote intestinal dysmotility and systemic inflammation
[6].
Liver sinusoidal endothelial cells (LSECs) may act as a hepatic barrier along the liver–gut axis. This is explained by the fact that LSECs are the first in contact with portal-delivered, gut-derived pathogens. LSECs contribute to the uptake and clearance of viruses, bacteriophages, microbial products, and metabolic wastes
[14].
Finally, a less-known communication route is a retrograde transit along enteric neurons, which can spread microbes that leak through the intestinal barrier
[9].
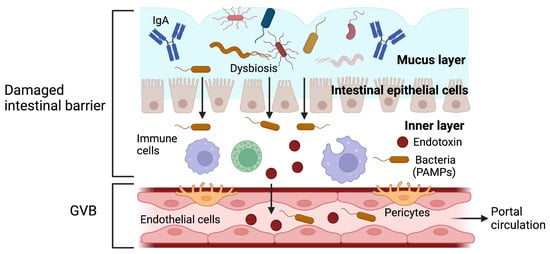
In the liver, the intestinal barrier must remain intact because increased intestinal permeability facilitates the entry of pathogen-associated molecular patterns (PAMPs) into the portal circulation, thus triggering a proinflammatory cascade and causing hepatic inflammation
[4]. PAMPs can also activate stellate cells, which are involved in promoting and progressing liver fibrosis
[6][6] (Figure 2).
Figure 2. The function of the intestinal barrier after being damaged. Alteration of the intestinal barrier increases in intestinal permeability and facilitates the translocation of bacteria and endotoxins, which can damage the gut–vascular barrier (GVB). The entry of pathogens and pathogen-associated molecular patterns (PAMPs) into the portal circulation triggers an inflammatory response. Gut-derived PAMPs can bind to specific toll-like receptors in the liver and activate the proinflammatory pathways, which result in hepatic inflammation. Adapted from ref. [4]. Created by BioRender (accessed on 29 August 2023).
One hypothesis suggests that the alteration of the intestinal barrier and the increase in intestinal permeability can produce an inflammatory response, and it is also believed that the intestinal microbiota can modulate this inflammation. This has led to the concept wherein “leaky gut syndrome” and “dysbiosis” are related to each other and could be involved in the pathogenesis of some gastrointestinal and systemic disorders
[8].
3. Dysbiosis in Non-Alcoholic Fatty Liver Disease
Trillions of microbes, including bacteria, archaea, viruses, and eukaryotic microbes, colonize the gastrointestinal tract of the human body. The amounts of these microorganisms vary according to the site in the gastrointestinal tract, and the stomach and duodenum have 10–10
3 bacteria per gram of content; the small intestine (10
4–10
7) and large intestine (10
11–10
12) are the sites where the highest levels of microbes are found
[15]. The dominant phyla in the large intestine are
Firmicutes and
Bacteroidetes; however, other phyla, such as
Actinobacteria and
Proteobacteria, are also present
[5].
The colon also harbors essential pathogens, such as
Escherichia coli (
E. coli),
Campylobacter jejuni,
Salmonella enterica,
Vibrio cholerae, and
Bacteroides fragilis; however, these pathogens usually are found at deficient levels (<0.1% of the gut microbiome)
[15]. It is estimated that there are approximately 10
5–10
6 fungal cells per gram of feces. The main fungal phyla in the intestines are
Ascomycota and
Basidiomycota [13].
These microbes interact with each other and cells in the human body in complex and poorly understood ways. These interactions affect the host’s metabolism, shape immunity, and facilitate digestion and nutrient absorption, among other functions
[16].
These microorganisms interact with one another through mutualism, commensalism, and competition
[13]. Furthermore, bacteria in a healthy gut are in homeostatic balance with their host and contribute to maintaining a healthy state. Dysbiosis is an imbalance or change in bacterial content or metabolic function
[15].
Several factors can modify the composition and function of the intestinal microbiota, including the host’s genetics, diet, age, antibiotics, and smoking. In addition, changes in the intestinal microbiota may contribute to developing diseases, such as NAFLD
[5].
A decrease in microbial gene richness (MGR) is associated with a proinflammatory state, adiposity with abdominal distribution, and a propensity for metabolic alterations, all related to the pathophysiology of NAFLD. People with a low MGR show an increase in bacteria capable of synthesizing lipopolysaccharides, which is related to insulin resistance and an adverse lipidomic profile, a part of the pathophysiology of NAFLD
[17]. Some studies have identified metabolites derived from gut bacteria that may be involved in developing hepatic steatosis
[13].
Patients with NAFLD and NASH show increased numbers of
Bacteroidetes and changes in the presence of
Firmicutes, resulting in a decreased F/B ratio. However, owing to the different molecular methods used to identify bacteria, F/B ratios can vary and produce inconsistent results. Patients with NAFLD have a higher proportion of
Clostridium,
Anaerobacter,
Streptococcus,
Escherichia, and
Lactobacillus and a lower proportion of
Oscillibacter,
Flavonifaractor,
Odoribacter, and
Alistipes spp. (
Table 1)
[18]. They show an increase in
Proteobacteria and
Enterobacteriaceae, along with a decrease in
Rikenellaceae and
Rumminoccaceae [17].
Table 1. Microbiota in healthy subjects vs. NAFLD according to genus.
| Increased |
Diminished |
| Lactobacillus |
Alistipes spp. |
| Robinsoniella |
Prevotella |
| Roseburia |
Odoribacter |
| Dorea |
Flavonifractor |
| Anaerobacter |
Oscillibacter |
| E. coli |
|
| Clostridium XI |
|
| Streptococcus |
There is evidence of a decrease in viral diversity and the proportion of bacteriophages in patients with NAFLD. The gut microbiome is affected in the early stages of the disease and is altered at later stages
[13].
In advanced fibrosis (F3–F4), an increase in Gram-negative microorganisms, a decrease in
Firmicutes, and a greater number of
Proteobacteria have been documented.
Escherichia coli and
Bacteroides vulgatus are the most abundant bacteria at the species level. Patients with NAFLD cirrhosis show more abundance of species within the
Enterobacteriaceae family and
Streptococcus genera
[17].
Individuals with NAFLD exhibit increased bacterial overgrowth in the small intestine and impaired intestinal permeability
[9]. Therefore, numerous therapeutic options have been proposed to modulate the composition and function of intestinal microbiota, including probiotics, prebiotics, and fecal microbiota transplantation (FMT)
[5].
4. Prebiotics and Probiotics in Non-Alcoholic Fatty Liver Disease
Probiotics are live microorganisms that can confer a health benefit on the host; the main genera of probiotics studied are
Lactobacillus and
Bifidobacterium. In turn, prebiotics are non-viable food components associated with microbiota modulation and can confer a health benefit on the host. Prebiotics consist primarily of polysaccharides (inulin, cellulose, hemicellulose, pectins, and resistant starch) and oligosaccharides (fructooligosaccharides, galactooligosaccharides, isomaltooligosaccharides, xylooligosaccharides, lactulose, and soy oligosaccharides), which stimulate the growth of beneficial bacteria. The most studied prebiotics in patients with NAFLD are fructooligosaccharides. Finally, synbiotics are a mix of probiotics and prebiotics
[19][20][21][19,20,21].
Different mechanisms of action have been proposed by which probiotics and prebiotics could benefit patients with NAFLD. These exert beneficial effects by altering the composition of the microbial flora. Probiotics can act in different target organs by producing antimicrobial peptides, reducing intestinal permeability, or preventing the translocation of bacterial products
[12].
Lactobacillus and
Bifidobacterium have been reported to be associated with ß-glucuronidase inhibition
[19]. At the same time,
Bifidobacterium protects against proinflammatory cytokine secretion and intestinal barrier dysfunction
[22].
Probiotics have positive effects on inflammatory liver damage mediated by c-Jun N-terminal kinase (JNK) and Nuclear Factor kappa B (NF-κB), which was correlated with Tumor Necrosis Factor-alpha (TNF-α) regulation and insulin resistance
[6].
Studies using prebiotics in NAFLD are limited compared to probiotics
[21]. Prebiotics can selectively promote the proliferation and activity of intestinal microbes. Animal models have shown that prebiotic supplementation reduces the fatty acid synthesis pathway, which may decrease fructose-induced hepatic triglyceride (TG) accumulation; this could be due to reduced gene expression of enzymes that regulate hepatic lipogenesis, such as acetyl Co-A carboxylase and fatty acid synthase. In addition, oligofructose modifies intestinal microbiota in favor of
Bifidobacterium, which improves mucosal barrier function and reduces the level of endotoxins
[12].
Administration of prebiotics could reduce liver inflammation through a glucagon-like peptide 2-dependent effect on the intestinal barrier; however, further studies are still needed to determine the mechanisms by which they could be beneficial in NAFLD
[6].
Finally, postbiotics are a broad range of bioactive molecules, including non-viable microbial cells, cell compounds, and any soluble products or metabolic by-products resulting from microorganisms, which confer a health benefit on the host. Postbiotics have been recognized to mimic the functions and activities of probiotics. These have not been directly evaluated in patients with NAFLD; however, their antioxidant, anti-obesogenic, anti-inflammatory, and anti-adipogenesis effects have been studied, as well as their action on insulin resistance
[23].