Telomeres are long non-coding regions found at the ends of eukaryotic linear chromosomes. Although they have traditionally been associated with the protection of linear DNA ends to avoid gene losses during each round of DNA replication, the role of these sequences and their adjacent regions go beyond just protecting chromosomal ends. Regions nearby to telomeric sequences have now been identified as having increased variability in the form of duplications and rearrangements that result in new functional abilities and biodiversity.
- telomere
- sub-telomere
- Aspergillus fumigatus
- Candida albicans
- Candida glabrata
- Pneumocystis jirovecii
1. Introduction
In eukaryotes, telomere sequences generally consist of double-stranded (ds) region composed of 5′-TTAGGG-3′ tandem repeats that end with a single-stranded (ss) 3′ overhang (Figure 1; [1][2][3]) Telomere maintenance can also be achieved through alternative lengthening of telomeres (ALT) pathways [4]. Various protein complexes have been found to interact with the telomeres directly or indirectly [5][6]. In humans, the core protein complex is referred to as Shelterin and it is composed by six proteins: Trf1, Trf2, Rap1 [7] , Tin2 [8], Tpp1 [9][10], and Pot1. Trf1 and Trf2 each bind the sequence 5′-YTAGGGTTR-3′ in duplex DNA, showing very low tolerance for single-base changes, while Pot1 has strong sequence specificity, binding single-stranded 5′-(T)TAGGGTTAG-3′ sites both at the 3′ end and at internal positions. Since these three shelterin subunits are connected through protein-protein interactions, shelterin has the capacity to recognize telomeric DNA with at least five DNA-binding domains (two each in Trf1 and Trf2 and one in Pot1) [5][11][12][13]. Furthermore, three other proteins, Ctc1, Stn1, and Ten1, forming the CST complex, binding single-stranded DNA at the telomeres have been identified [14]. As a consequence, shelterin is uniquely qualified to distinguish telomeres from all other DNA ends.
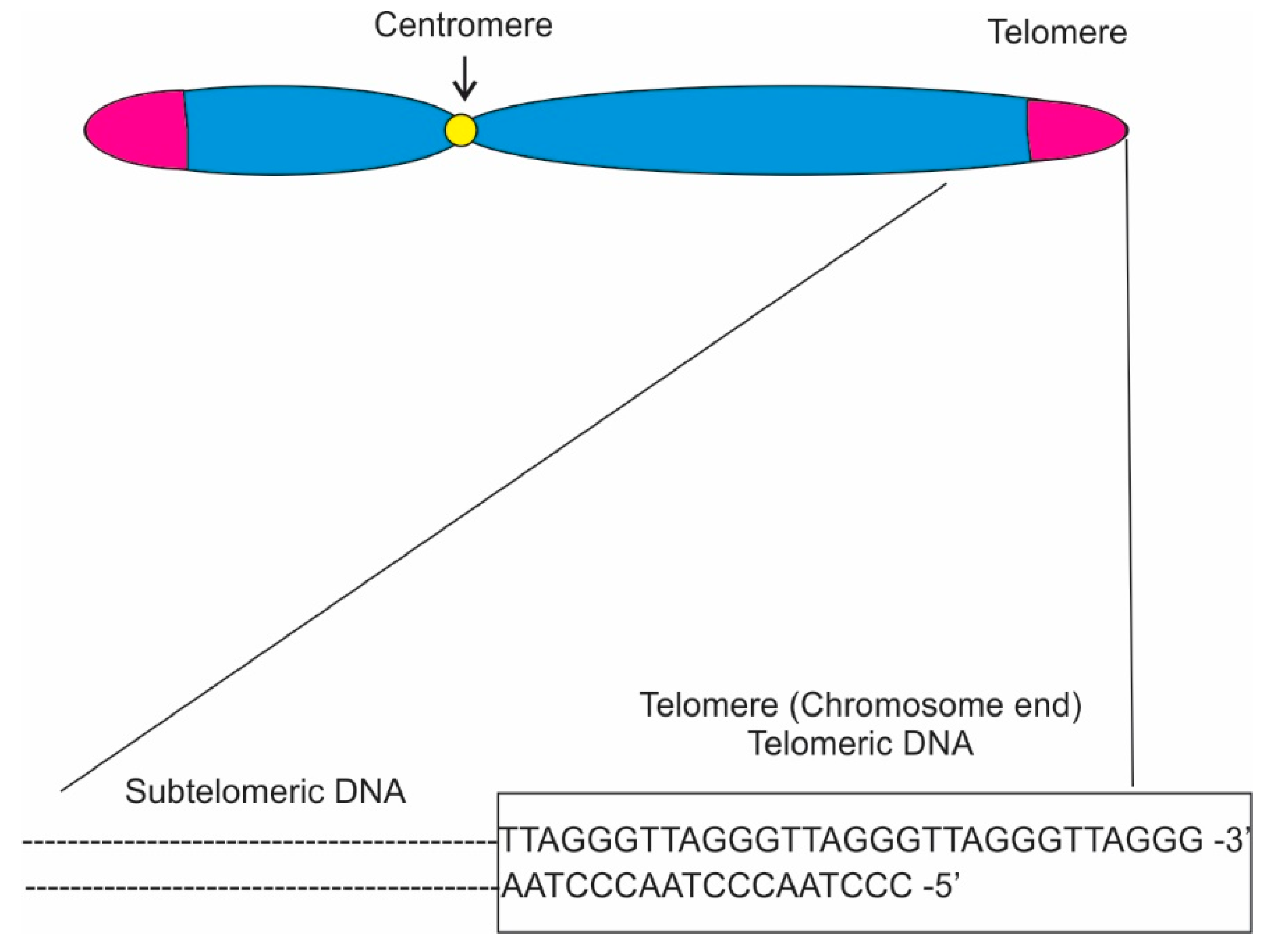
Shelterin-related complexes are also found at telomeres in other eukaryotes. Pot1-like proteins are present in nearly all eukaryotes, a Trf1/2-like protein is found in fission yeast and in trypanosomes. Telomeric proteins have been identified and characterized in yeast, such as fission yeast’s Rap1, which is a homologue of the mammalian protein, or Tpz1, Taz1, and Pot1, which are orthologs of the mammalian shelterin proteins Tpp1, Trf1/2, and Pot1. The role of these proteins encompasses regulation of telomere length and chromosome end protection, but most important for specific gene expression, they have a role in gene silencing. In Saccharomyces cerevisiae , for example, Rap1 recruits both the Sir complex (Sir 1, 2, and3) and Rif1 and Rif2. While Rif1 and Rif2 have a role in telomere length, the Sir complex is critical for gene silencing at telomeres and adjacent subtelomeric regions.
Gene silencing is a critical mechanism in fungal cells with subtelomeric regions adjacent to telomere being frequent targets of transcriptional repression via epigenetic regulators, such as histone deacetylases and methyltransferases [15]. Methyltransferases not only serve as recruitment and recognition sites for further repressive proteins, but also inhibit acetylation to promote a gene-silencing cascade [15]. Even though gene silencing may sound alarming, it has actually been found to be an invaluable mechanism at telomeric and subtelomeric regions, as this silencing appears to help maintain telomeric chromatin stability and prevents telomere-dependent cellular senescence through the prevention of decreased telomere lengths [15].
Sub-telomeric regions are also of great interest as pathogens can exploit the telomere position effect (TPE), an event that involves the silencing of genes located near the telomeric chromatin, to regulate gene expression. For example, TPE regulates the expression of secondary metabolites, cell surface proteins, such as adhesins, and other components that can support the organisms during the transition to pathogen. Many of these putative genes, or gene clusters, found at sub-telomeric regions on the chromosomes have been shown to be under epigenetic regulation, which may allow the organism to tailor its behavior to specific growth conditions [16]. Although telomeric instability due to issues with any of the telomeric proteins is generally correlated to the onset of cancer in humans and has a lethal effect on less complex organism, chromosomal rearrangements at the sub-telomeric regions may increase genomic plasticity and lead to increased virulence and drug resistance [17].
2. Aspergillus fumigatus
Telomeres in A. fumigatus are comprised of 7–21 tandem repeats of the common eukaryotic sequence TTAGGG (Table 1; [18]). Similar to the other fungi listed in this research, changes in the transcriptional expression of specific genes during infection affects the virulence and pathogenicity of A. fumigatus. Various candidate genes have been identified and the wide array of functions points to a complex picture. One hypothesis is that fungi secondary metabolites, which are generally organized in clusters, and tend to be species specific, could have a role in virulence. In A. fumigatus, there are 26 such clusters. A few of the clusters are unique to A. fumigatus and diverge from comparable family members, A. oryzae and A. nidulans, and others are missing. The location of these clusters of diverged and/or missing gene clusters shows a bias towards telomeric/subtelomeric locations [18] supporting the suggestion of greater genomic instability at these regions.
Table 1. Telomere repeat sequences and base pair length for all four opportunistic fungi.
| Fungal Species | Telomere Repeat Sequence | Number of bp Repeats |
|---|---|---|
| Aspergillus fumigatus | 5′-TTAGGG-3′ | 6 |
| Candida albicans | 5′-ACTTCTTGGTGTACGGATGTCTA-3′ | 23 |
| Candida glabrata | 5′-CTGGGTGCTGTGGGGT-3′ | 16 |
| Pneumocystis jirovecii | 5′-TTAGGG-3′ | 6 |
Comparison between the genes under LaeA control [19] and the ones expressed during early-stage infection in murine models [20] confirmed alignment of 99 genes out of 415 with 40% coding for secondary metabolites, including pseurotin, confirming the role of LaeA in A. fumigatus infection. Sub-telomeric genes were also preferentially expressed during nitrogen starvation indicating a role for sub-telomeric genes during nutrient starvation [20].
A recent paper investigating the shift of A. fumigatus to the biofilm phenotype identified a gene, biofilm architecture factor A ( bafA ), as one of the main players in the shift. bafA is part of a larger family of genes that can modulate the morphological changes needed to decrease the presence of vertically oriented hyphae and an increase in the ability of A. fumigatus to survive and thrive in a low oxygen environment, with a corresponding increase in the organism virulence. Similar to other genes involved in the organism pathogenicity, bafA is located within a subtelomeric gene cluster (Table 2; [21]).
Table 2. Subtelomeric gene cluster members that increase pathogenicity in A. fumigatus.
| Gene/Genes in Subtelomeres | Protein/Metabolite | Function | References |
|---|---|---|---|
| Fumitremorgin biosynthesis supercluster | Pseurotin | Neuritogenic, nematicidal quinone | [20][22] |
| LaeA gene | LaeA methyltransferase | Global transcriptional regulator of secondary metabolite biosynthesis; virulence factor | [23] |
| bafA | Biofilm Architecture Factor A | Modulates hyphae formation and ability to survive low oxygen conditions increasing pathogenicity | [21] |
| atmA and atrA | Ataxia-telangiectasia mutated kinases | Chromosomal polymorphism | [24] |
Further studies will be needed to characterize the structure and function of gene clusters associated with virulence. New strategies, such as updated recombineering protocols [25] are being explored to overcome the technical difficulties involved in genetic manipulation of A. fumigatus . The sub-telomeric localization of many of the clusters suggests a role for epigenetic regulation of the genes, such as TPE (telomere position effect), which can effect gene expression [26]. It is conceivable that this strategy is used by the pathogen to tailor secondary metabolites synthesis to specific growth conditions. In addition, the diversity of genes found at telomeric and sub-telomeric regions may be due to the fact that they are hotspots for recombination events as it has been observed in many other organisms, for example, with chromosomal breaks followed by telomerase-mediated healing. In fact, in ΔatmA and ΔatrA mutants, some chromosomal polymorphism was observed suggesting their possible role in telomeric and subtelomeric maintenance (Table 2; [24]).
3. Candida albicans
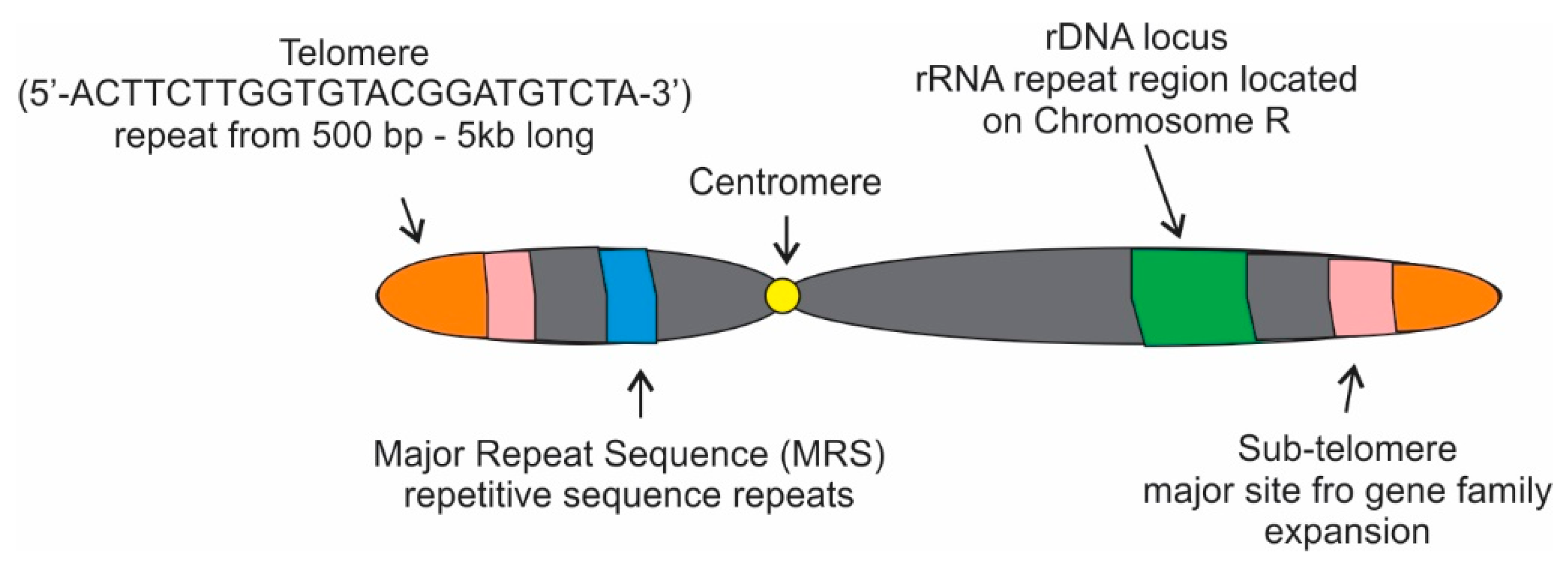
Four major repetitive regions are present in the C. albicans genome: telomeres, sub-telomeres, the Major Repeat Sequence ( MRS ), and the rDNA locus (Figure 2; [27]). The telomeres of C. albicans have gained attention throughout the years for various reasons including their unusually long length [28]. These telomeres are composed of long tandem copies of a 23-bp repeat (5′-ACTTCTTGGTGTACGGATGTCTA-3′) that has considerably diverged from other Candida spp. with respect to both length and complexity [27]. Of the four fungal strains focused on in this resviearchw, Candida ’s extended length becomes most obvious when compared to the standard TTAGGG sequence that is the most common telomere repeat in the animal and fungi kingdoms (Table 1; [29]). Normally, the repeat copy numbers of that 23-bp repeat are more than 20 copies per haploid genome and the size is about 500 bp to 5 kb.
A variety of significant regulatory regions in telomeres can influence pathogenicity and viability. Telomerase complex, which contains telomerase reverse transcriptase and telomerase RNA component, can maintain the length of telomere to prevent cell senescence via the production and maintenance of the 23 bp repeats at the telomeres [27]. Both telomerase RNA component and telomerase reverse transcriptase, encoded by TER1 and EST2 (ever shorter telomeres 2), respectively, perform repeated rounds of reverse transcription to build G overhangs as part of the end-protective T-loops that prevent telomere attrition in some fungal species (Table 3; [27][30]). More specifically, Est2 uses the TER1 RNA as a template to add telomeric repeat subunits to the chromosome end in order to maintain replicative capacity and avoid cell senescence. The dual roles of end protection and telomere repeat addition by Est2 are separable. A catalytically inactive Est2 retains the ability to suppress excessive G-strand accumulation despite being unable to add telomeric repeats.
Within C. albicans , Est3 has been found to be an essential subunit of the telomerase regulatory complex with similarities to the telomeric protein, Tpp1, of mammalian cells (Table 3; [31][32]. It has been demonstrated that Est3 have a telomerase-stimulatory function required for telomerase activity [32]. Rap1 (repressor activator protein 1) is also critical for telomeres in C. albicans [33]. In many fungi, such as S. cerevisiae , Rap1 is required for cell viability. However, in C. albicans , it has been found that Rap1 is not required for viability, and instead functions as a critical regulator of telomeric length and structure [33]. RAP1 is also conserved, with a human ortholog, hRAP1 in existence [7]. Rap1 has been shown to have an unusually small size in C. albicans , and even lacks the usual C-terminal domain found in other fungal Rap1 proteins [33]. C. albicans’ null mutants lacking RAP1 are viable, but exhibit slowed growth and abnormal filament morphologies similar to those that would be observed in DNA repair mutants. These rap1 null strains also exhibit unusually long telomeres with a complete lack of regulated length control. It has been shown that Rap1 works in conjunction with a series of other telomeric proteins, including Ku70, Stn1, and Ten1, to suppress abnormal recombination and maintain telomeres (Table 3 ; [33]).
Table 3. Subtelomeric genes that increase pathogenicity in C. albicans.
| Gene/Genes | Protein/Metabolite | Function | References |
|---|---|---|---|
| TER1 | Telomerase RNA component | Involved in production and maintenance of telomeres to prevent cell senescence | [27][30] |
| EST2 | Telomerase reverse transcriptase | Involved in production and maintenance of telomeres to prevent cell senescence | [27][30] |
| EST3 | Subunit of telomerase regulatory complex | Telomerase stimulatory function | [31][32] |
| RAP1 | Repressor activator protein 1 | Regulator of telomere length and structure; works in conjunction with Ku70, Stn1, and Ten1 | [33] |
| TLO genes | Highly variable Telomere associated metabolites | Includes Med2 and other subunit components of mediator complex and transcriptional responses; Involved in adaptability, hyphal growth, and oxidative stress responses | [34][35] |
Amongst these variable sub-telomeric sequences, TLO genes (telomere-associated genes) have been expanded and are linked to the virulence and adaptability of Candida albicans as a pathogen ( Table 3; [35]). Whereas other Candida species have one or two TLO genes, including the closely related C. dubliniensis , C. albicans has diverged to have a striking 14 to 15 TLO genes. In C. albicans , as the name suggests, the TLO genes are situated close to the telomeres of each chromosome. Three clades (α, β, and γ) of these expressed TLO genes have been identified. They all contain a Med2 domain, but the difference is based upon long terminal repeat insertions sequences [35]. Med2 is a component of the Mediator complex [34]. Mediator is a large multi-subunit protein complex, which is conserved throughout eukaryotes, and mediates interaction between RNA polymerase II, and the machinery used in the initiation of transcription at target gene promoters [34]. It has been shown that the deletion of the TLO regions in C. dubliniensis , a close relative to C. albicans , resulted in the loss of virulence through hindered hyphal growth and stress responses [34]. This is due to the defects in activation of transcriptional responses associated with a number of virulence traits including tolerance of oxidative stress and hypha formation. In C. albicans , hyphal growth, and increased adaptability are amongst the traits considered as contributing to its increased virulence compared to other Candida species [35]. It has been suggested that the expanded TLO regions with broad variability in expression levels and localization, can contribute to subsequent variability in the Mediator complex subunits and, thus, promotes increased adaptability and virulence [34][35].
4. Candida glabrata
Although not much is known about the factors that facilitate C. glabrata virulence and pathogenicity, like for other fungi, it is believed that the ability to strongly adhere to host cells and other surfaces is a critical contributing factor. In vitro, C. glabrata adherence to epithelial cells is mediated by a large adhesin, Epa1, which is generally expressed in vitro during lag phase and is repressed during log and stationary phase [36][37]. Epa1 belongs to a broad class of glycosylphosphatidylinositol-anchored cell wall proteins (GPI-CWPs) found in other species =, such as S. cerevisiae , C. albicans , and A. fumigatus (Table 4). Various studies showed that EPA1 is part of a larger family of adhesin genes in C. glabrata, and that, like for other pathogens, including P. jirovecii, the cluster of genes shows a sub-telomeric localization and it is regulated through sub-telomeric silencing mechanisms [38][39][40]. De Las Peñas et al. , [39] characterized EPA2 - EPA5 with the genes divided in two clusters, EPA1 - EPA3 and EPA4 - EPA5 , found at sub-telomeric regions. Deletion of the two different clusters of genes shows a moderate decrease in colonization, specifically of the kidneys, but altogether only showed a small effect on the yeast virulence. Interestingly, however, the only gene expressed in the various in vitro conditions was EPA1 , indicating that the rest of the members are generally silenced. Hyper-adherent mutants allowed for the isolation of two novel EPA genes, EPA6 and EPA7 [40]. The two adhesins were also found while exploring factors involved in C. glabrata biofilm formation [38]. In all cases, the regulation of expression of the genes was found to be through the sub-telomeric silencing machinery.
Phylogenetically, C. glabrata is more closely related to S. cerevisiae than to C. albicans , as close homologues of S. cerevisiae genes can be found in the C. glabrata genome and both genomes conserve a high degree of synteny [41][42]. Considering the reported synteny between genes in the two organisms, it has been explored if similar sub-telomeric silencing complexes were acting in both organisms. The silencing of the adhesin genes is, in fact, mediated by proteins similar to the machinery used for telomeric silencing in S. cerevisiae; however, interestingly enough, the family cluster of adhesin genes are not found in S. cerevisiae [39] and the highly homologous silencing complex proteins seem to have different roles in the two organisms [38]. C. glabrata has in effect homologues of all the S. cerevisiae genes implicated in transcriptional silencing at the telomeres barring SIR1 . Silencing in S. cerevisiae is mediated by binding of RAP1 to the telomeric repeats. The bound protein is known to recruit the SIR complex. One of the components of the SIR family, the histone deacetylase SIR2, which targets H3 and H4, primes the region increasing the binding of two other components, SIR3 and SIR4 and extending the transcriptional repression (Table 4). Another sets of proteins RAP1 interacts with are RIF1 and RIF2, which are involved in telomere length regulation, with RIF1 having a role in the silencing of both the telomeric and sub-telomeric regions (Table 4). Both sir3Δ strains and strains with a mutated RAP1 de-repressed the transcription of the EPA2-5 genes, albeit silencing differed for the two different clusters with a complete silencing for EPA4/5, and a more gradual telomere-distance dependent silencing for the EPA1-4 cluster [39]. In the C. glabrata hyper-adherent mutants increased adherence was also affected by EPA genes, and RIF1 and SIR3 . sir3 Δ and rif1 Δ strains showed induction of genes from the whole EPA family, with EAP1 , EPA6, and EPA7 being the main, albeit not the only, drivers of the hyper-adherent phenotype. RIF1 , RAP1, and SIR3 are required in C. glabrata for the silencing of genes at sub-telomeric locations with silencing generally decreasing as the distance from the telomere increases.
Table 4. Subtelomeric genes that increase pathogenicity in C. glabrata.
| Gene/Genes | Protein/Metabolite | Function | References |
|---|---|---|---|
| EPA1–7 | Large adhesin proteins (glycosylphosphatidylinositol-anchored cell wall protein) | Adherence; clusters of subtelomeric EPA genes are involved in colonization and biofilm formation | [36][37] |
| SIR 1–4 | Histone deacetylase and silencing proteins | Involved in transcriptional repression, gene silencing, and adhesion | [39] |
| RAP1 | Repressor activator protein 1 | Regulator of telomere length and structure; Involved in gene silencing | [39] |
| RIF1–2 | Rif1 and Rif2 regulatory Proteins | Regulators of telomere length and structure; Involved in gene silencing | [43] |
| HDF1–2 | yKu70/80 | Telomere maintenance; non-homologous end join repair | [44] |
The silencing and release of repression are the basis of the different expression of the adhesin family members in the various mutant backgrounds. These results suggest that their transcription is likely mediated by the sub-telomeric silencing machinery in response or in addition to responses to particular environmental clues. Rosas-Hernandez et al. , [44] looked at the role of the proteins in the various telomeric complex for specific telomeres in C. glabrata and found that silencing of different sub-telomeric regions is based on different requirements. They established that HDF1 and HDF2, coding for yKu70/80, are functional equivalents of the S. cerevisiae genes and have a role in telomere maintenance and non-homologous end join repair (NHEJ) (Table 4). They also found that although Rap1, Sir2, Sir3, and Sir4 are required for sub-telomeric genes silencing at various telomeres, Rif1, yKu70/80 function has a different impact at different sub-telomeric regions. In C. glabrata, the sub-telomeric regions subjected to TPE can reach up to 25 kb, much larger than in other known fungi, and SIR2 , SIR3 , and SIR4 are required for TPE to work. Rif1, yKu70, and yKu80, however, do not have such blanket effect. yKu70 and yKu80 are required for TPE at some chromosomes, but not all. Rif1 has a positive role in telomeres silencing but its effect varies at different telomeres and shows discontinuous silencing across the telomere. The differential effect of these proteins on the telomeres may be due to the presence of cis -acting silencers at sub-telomeric regions. A silencer regulating EPA3 was isolated in C. glabrata with its function based on interactions with Sir3. Its function, based on data from silencers in S. cerevisiae , is correlated to telomeres length and consequent availability of Sir3. Longer telomeres, like in rif1 Δ, would mean more Rap1 bound to the DNA and greater recruitment of the SIR complex to telomeres and less to the cis -element and hence de-repression of the gene expression. Shorter telomeres, like in hdf1 Δ and hdf 2Δ, and more availability of Sir3, results in stronger silencing of the sub-telomeric genes at that region [44]. The complex regulation of expression of adhesin genes with differential silencing and expression of the proteins at different telomeres in response to environmental clues can be the basis of the antigen heterogenicity on different cells in the same population and guarantee an escape mechanism for the pathogen during infections in vivo.
yKu70, yKu80, and Rif1 are also involved in sub-telomeric silencing of the MTL3 locus in C. glabrata. Although C. glabrata has never been shown to mate and in general human pathogenic fungi do not show a sexual cycle, it has been hypothesized that mating could activate specific combinations of genes required for virulence and survival in the host. The MTL3 silencing is imperfect and can potentially have a role in cell-specific genes seen in C. glabrata that could be correlated with pathogenicity and virulence [45].
References
- Červenák, F.; Sepšiová, R.; Nosek, J.; Tomáška, Ľ. Step-by-Step Evolution of Telomeres: Lessons from Yeasts. Genome Biol. Evol. 2021, 13, 1–12.
- Mceachern, M.J.; Blackburn, E.H. A conserved sequence motif within the exceptionally diverse telomeric sequences of budding yeasts. Proc. Natl. Acad. Sci. USA 1994, 91, 3453–3457.
- Fulnečková, J.; Ševčíková, T.; Fajkus, J.; Lukešová, A.; Lukeš, M.; Vlček, Č.; Lang, B.F.; Kim, E.; Eliáš, M.; Sýkorová, E. A broad phylogenetic survey unveils the diversity and evolution of telomeres in eukaryotes. Genome Biol. Evol. 2013, 5, 468–483.
- Nabetani, A.; Ishikawa, F. Alternative lengthening of telomeres pathway: Recombination-mediated telomere maintenance mechanism in human cells. J. Biochem. 2011, 149, 5–14.
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110.
- Diotti, R.; Loayza, D. Shelterin complex and associated factors at human telomeres. Nucleus 2011, 2, 119–135.
- Li, B.; Oestreich, S.; De Lange, T. Identification of human Rap1: Implications for telomere evolution. Cell 2000, 101, 471–483.
- Kim, S.H.; Kaminker, P.; Campisi, J. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 1999, 23, 405–412.
- Liu, D.; Safari, A.; O’Connor, M.S.; Chan, D.W.; Laegeler, A.; Qin, J.; Songyang, Z. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 2004, 6, 673–680.
- Ye, J.Z.S.; Hockemeyer, D.; Krutchinsky, A.N.; Loayza, D.; Hooper, S.M.; Chait, B.T.; De Lange, T. POT1-interaction protein PIP1: A telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 2004, 18, 1649–1654.
- Broccoli, D.; Smogorzewska, A.; Chong, L.; de Lange, T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 1997, 17, 231–235.
- Baumann, P.; Cech, T.R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 2001, 292, 1171–1175.
- Červenák, F.; Juríková, K.; Sepšiová, R.; Neboháčová, M.; Nosek, J.; Tomáška, L.U. Double-stranded telomeric DNA binding proteins: Diversity matters. Cell Cycle 2017, 16, 1568–1577.
- Miyake, Y.; Nakamura, M.; Nabetani, A.; Shimamura, S.; Tamura, M.; Yonehara, S.; Saito, M.; Ishikawa, F. RPA-like Mammalian Ctc1-Stn1-Ten1 Complex Binds to Single-Stranded DNA and Protects Telomeres Independently of the Pot1 Pathway. Mol. Cell 2009, 36, 193–206.
- Jezek, M.; Gast, A.; Choi, G.; Kulkarni, R.; Quijote, J.; Graham-Yooll, A.; Park, D.; Green, E.M. The histone methyltransferases Set5 and Set1 have overlapping functions in gene silencing and telomere maintenance. Epigenetics 2017, 12, 93–104.
- Scharf, D.H.; Heinekamp, T.; Brakhage, A.A. Human and Plant Fungal Pathogens: The Role of Secondary Metabolites. PloS Pathog. 2014, 10, e1003859.
- Poláková, S.; Blume, C.; Zárate, J.Á.; Mentel, M.; Jørck-Ramberg, D.; Stenderup, J.; Piškur, J. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. Proc. Natl. Acad. Sci. USA 2009, 106, 2688–2693.
- Nierman, W.C.; Pain, A.; Anderson, M.J.; Wortman, J.R.; Kim, H.S.; Arroyo, J.; Denning, D.W. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 2005, 438, 1151–1156.
- Perrin, R.M.; Fedorova, N.D.; Bok, J.W.; Cramer, R.A., Jr.; Wortman, J.R.; Kim, H.S.; Nierman, W.C.; Keller, N.P. Transcriptional Regulation of Chemical Diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007, 3, 508–517.
- McDonagh, A.; Fedorova, N.D.; Crabtree, J.; Yu, Y.; Kim, S.; Chen, D.; Loss, O.; Cairns, T.; Goldman, G.; Armstrong-James, D.; et al. Sub-telomere directed gene expression during initiation of invasive Aspergillosis. PLoS Pathog. 2008, 4, e1000154.
- Kowalski, C.H.; Morelli, K.A.; Stajich, J.E.; Nadell, C.D.; Cramer, R.A. A heterogeneously expressed gene family modulates biofilm architecture and hypoxic growth of Aspergillus fumigatus. mBio 2020.
- Doyle, S.; Jones, G.W.; Dolan, S.K. Dysregulated gliotoxin biosynthesis attenuates the production of unrelated biosynthetic gene cluster-encoded metabolites in Aspergillus fumigatus. Fungal Biol. 2018, 122, 214–221.
- Lind, A.L.; Lim, F.Y.; Soukup, A.A.; Keller, N.P.; Rokas, A. An LaeA-and BrlA-dependent cellular network governs tissue-specific secondary metabolism in the human pathogen Aspergillus fumigatus. Msphere 2018, 3, e00050-18.
- Dos Reis, T.F.; Silva, L.P.; de Castro, P.A.; de Lima, P.B.A.; do Carmo, R.A.; Marini, M.M.; da Silveira, J.F.; Ferreira, B.H.; Rodrigues, F.; Malavazi, I.; et al. The influence of genetic stability on Aspergillus fumigatus virulence and azole resistance. G3 Genes Genom. Genet. 2018, 8, 265–278.
- Igarashi, Y.; Bowyer, P.; Bignell, E.; Alcazar-Fuolia, L.; Cairnsb, T.; Lopez, J.F.; Zonja, B.; Pérez, S.; Barceló, D. A modified recombineering protocol for the genetic manipulation of gene clusters in Aspergillus fumigatus. PLoS ONE 2014, 9, e111875.
- Palmer, J.M.; Keller, N.P. Secondary metabolism in fungi: Does chromosomal location matter? Curr. Opin. Microbiol. 2010, 13, 431–436.
- Dunn, M.J.; Anderson, M.Z. To repeat or not to repeat: Repetitive sequences regulate genome stability in Candida albicans. Genes 2019, 10, 866.
- McEachern, M.J.; Hicks, J.B. Unusually large telomeric repeats in the yeast Candida albicans. Mol. Cell. Biol. 1993, 13, 551–560.
- Underwood, A.P.; Louis, E.J.; Borts, R.H.; Stringer, J.R.; Wakefield, A.E. Pneumocystis carinii telomere repeats are composed of TTAGGG and the subtelomeric sequence contains a gene encoding the major surface glycoprotein. Mol. Microbiol. 1996, 19, 273–281.
- Hsu, M.; McEachern, M.J.; Dandjinou, A.T.; Tzfati, Y.; Orr, E.; Blackburn, E.H.; Lue, N.F. Telomerase core components protect Candida telomeres from aberrant overhang accumulation. Proc. Natl. Acad. Sci. USA 2007, 104, 11682–11687.
- Lee, J.W.; Ong, E.B.B. Genomic Instability and Cellular Senescence: Lessons from the Budding Yeast. Front. Cell Dev. Biol. 2020, 8, 619126.
- Yu, E.Y.; Wang, F.; Lei, M.; Lue, N.F. A proposed OB-fold with a protein-interaction surface in Candida albicans telomerase protein Est3. Nat. Struct. Mol. Biol. 2008, 15, 985.
- Yu, E.Y.; Yen, W.F.; Steinberg-Neifach, O.; Lue, N.F. Rap1 in Candida albicans: An unusual structural organization and a critical function in suppressing telomere recombination. Mol. Cell. Biol. 2010, 30, 1254–1268.
- Haran, J.; Boyle, H.; Hokamp, K.; Yeomans, T.; Liu, Z.; Church, M.; Fleming, A.B.; Anderson, M.Z.; Berman, J.; Myers, L.C.; et al. Telomeric ORFs (TLO s) in Candida spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits. PLoS Genet. 2014, 10, e1004658.
- Anderson, M.Z.; Baller, J.A.; Dulmage, K.; Wigen, L.; Berman, J. The three clades of the telomere-associated TLO gene family of Candida albicans have different splicing, localization, and expression features. Eukaryot. Cell 2012, 11, 1268–1275.
- Cormack, B.P.; Ghori, N.; Falkow, S. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 1999, 285, 578–582.
- Gallegos-García, V.; Pan, S.J.; Juárez-Cepeda, J.; Ramírez-Zavaleta, C.Y.; Martin-del-Campo, M.B.; Martínez-Jiménez, V.; Castaño, I.; Cormack, B.; de Las Peñas, A. A novel downstream regulatory element cooperates with the silencing machinery to repress EPA1 expression in candida glabrata. Genetics 2012, 190, 1285–1297.
- Iraqui, I.; Garcia-Sanchez, S.; Aubert, S.; Dromer, F.; Ghigo, J.M.; D’Enfert, C.; Janbon, G.; Enfert, C.; Janbon, G. The Yak1p kinase controls expression of adhesins and biofilm formation in Candida glabrata in a Sir4p-dependent pathway. Mol. Microbiol. 2005, 55, 1259–1271.
- De Las Peñas, A.; Pan, S.J.; Castaño, I.; Alder, J.; Cregg, R.; Cormack, B.P. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003, 17, 2245–2258.
- Castaño, I.; Pan, S.J.; Zupancic, M.; Hennequin, C.; Dujon, B.; Cormack, B.P. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol. Microbiol. 2005, 55, 1246–1258.
- Dujon, B.; Sherman, D.; Fischer, G.; Durrens, P.; Casaregela, S.; Lafentaine, I.; De Montigny, J.; Marck, C.; Talla, E.; Goffard, N.; et al. Genome evolution in yeasts. Nature 2004, 430, 35–44.
- Butler, G.; Rasmussen, M.D.; Lin, M.F.; Santos, M.A.S.; Sakthikumar, S.; Munro, C.A.; Rheinbay, E.; Grabherr, M.; Forche, A.; Reedy, J.L.; et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 2009, 459, 657–662.
- Shubin, C.B.; Greider, C.W. The role of Rif1 in telomere length regulation is separable from its role in origin firing. Elife 2020, 9, e58066.
- Rosas-Hernández, L.L.; Juárez-Reyes, A.; Arroyo-helguera, O.E.; De Las Peñas, A.; Pan, S.J.; Cormack, B.P.; Castaño, I.; Rosas-herna, L.L.; Jua, A.; Arroyo-helguera, O.E.; et al. yKu70/yKu80 and Rif1 regulate silencing differentially at telomeres in Candida glabrata. Eukaryot. Cell 2008, 7, 2168–2178.
- Ramírez-Zavaleta, C.Y.; Salas-Delgado, G.E.; de Las Peñas, A.; Castaño, I. Subtelomeric silencing of the MTL3 locus of Candida glabrata requires yKu70, yKu80, and Rif1 proteins. Eukaryot. Cell 2010, 9, 1602–1611.
