The melanocortin system is a complex set of molecular mediators and receptors involved in many physiological and homeostatic processes. These include the regulation of melanogenesis, steroidogenesis, neuromodulation and the modulation of inflammatory processes. In the latter context, the system has assumed importance in conditions of chronic digestive inflammation, such as inflammatory bowel diseases (IBD), in which numerous experiences have been accumulated in mouse models of colitis.
- melanocortin
- inflammatory bowel disease
- Crohn’s disease
- ulcerative colitis
- α-MSH
1. Introduction
2. α-MSH as a Key Melanocortin in the Modulation of Inflammatory Processes
The direct role of melanocortins in the regulation of inflammatory processes has emerged from their potential to inhibit the family of NF-κB involved in the transcriptional regulation of many genes involved in the synthesis of cytokines (especially TNF) and related receptors, chemokines and adhesion molecules [18,19,20][6][7][8]. NF-κB can exert its activity in the presence of an adaptor that gives it the potential to activate the transcriptional process (activating domain). However, some proteins in this family, such as NF-κB1,2, lack this domain. They, therefore, reside in the cytoplasm, connected to an inhibitor molecule, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IkBα). IkBα can be degraded, for example, by activating a series of receptors of the inflammatory system (such as TNFR, TLR, IL-1R), which, by promoting its degradation, allow NF-κB to translocate within the nucleus and act as a transcription factor [21][9]. NF-κB plays a role in both innate and acquired immunity. For example, it can be induced by toll-like receptors if they use myeloid differentiation primary response gene 88 (MyD88) as an adaptor. All toll-like receptors (TLR 1-9) are potential activators of MyD88, except for TLR3 [21,22][9][10]. α-MSH is a 13-amino-acid neuroendocrine peptide that may play a vital role in these processes. For example, it may be responsible for downgrading NF-κB and inhibiting IL-8 within endotoxin-stimulated monocytes and tumour necrosis factor (TNF) [23,24,25][11][12][13]. The effects of α-MSH are also exerted in the lymphocyte population. Evidence has shown its potential to induce immunological tolerance mechanisms and promote the development of CD4+ CD25+ T-regulatory lymphocytes. These regulatory T cells require antigen recognition for activation, but through nonspecific TGF-β1-mediated mechanisms, they can suppress other effector T cells, thus exhibiting immunomodulatory action [13,14][14][15]. Furthermore, several cytokines such as IL-2, IFN-γ and IL-10 are under the regulation of α-MSH within antigen-induced cell proliferation. Interleukin-10 inhibits other proinflammatory cytokines such as IL-2, IFN-γ and TNF-α, and α-MSH has been shown to reduce the antigen-induced proliferation of splenic cells and nonregulatory CD4+-CD25- lymphocytes [26][16]. Indeed, it appears that α-MSH may mediate the induction of TGF-β-producing T cells and suppressing the production of IFN-γ [27,28][17][18].3. The Receptors of the Melanocortin System: The Basis for Their Role in Peripheral and Systemic Inflammation
3.1. MC1R
MC1R is the main receptor involved in the mechanisms of melanogenesis and response to ultraviolet (UV) radiation [23,30][11][19]. Nevertheless, it has been implicated in several other homeostatic processes. Indeed, it is expressed within the vascular endothelium, acting not only in regulating vascular tone but also in preventing endothelial dysfunction following cell migration processes [31][20]. Several MC1R polymorphisms have been associated with a lower risk of complicated post-traumatic sepsis in preclinical models, suggesting its possible role in the post-traumatic inflammatory response [32][21]. In a mouse model of streptozotocin-induced diabetic retinopathy, the selective MC1R activation reduced the retinal vascular alterations and decreased the retinal proinflammatory cytokines and chemokines [33][22]. These anti-inflammatory actions were specifically observed in primary retinal cells from mice exposed to high glucose concentrations, with enhanced antioxidant levels and the preservation of photoreceptor integrity [34][23]..2. MC2R
3.2. MC2R
MC2R has been reported in some inflammatory pathways. Some authors wanted to evaluate the expression profiles of melanocortin receptors about the response to adalimumab in patients with rheumatoid arthritis. It was found that the expression of MC2R, MC3R and MC4R is reduced in CD8+ and CD19+ lymphocytes in anti-TNF-responsive patients after three months of follow-up [37][24]. However, the precise molecular mechanism underlying this modulation is still unknown.3.3. MC3R
Numerous experimental results have demonstrated the involvement of this receptor in inflammatory processes. Several molecules capable of interacting with MC3R have shown the anti-inflammatory properties of this receptor. MC3R agonists have been extensively studied in mouse models as potential therapeutic agents under the anti-inflammatory properties derived from MC3R activation. ACTH fragments capable of activating MC3R can inhibit cytokine synthesis in peritoneal macrophages, indirectly blocking neutrophilic diapedesis [38,39][25][26]. In addition, in the cardiac muscle, where macrophages express MC3R, the administration of agonists during acute myocardial infarction showed a protective role in mice, even during reperfusion, demonstrating that this protection was due to a reduction in systemic and local inflammatory markers (i.e., IL-1 and myeloperoxidase) [40][27].3.4. MC4R
MC4R is the primary melanocortin receptor present in the central nervous system [36][28]. It is predominantly expressed in the cerebral cortex, hypothalamus, thalamus, spinal cord and brainstem. Its distribution in the CNS is greater than MC3R and is generally nonoverlapping. Within the ventral periventricular and premammillary nuclei as well as in the posterior hypothalamic nucleus, however, coexpression of MC3R and MC4R can be observed [44][29]. Due to their localisation in the brainstem, melanocortins can activate several anti-inflammatory pathways via the efferent vagal pathway, reducing the expression of proinflammatory cytokines in endotoxemia, sepsis and other inflammatory conditions [45,46,47][30][31][32]. The concept of immunomodulatory efferent signalling of the vagus nerve has emerged. Cholinergic neurotransmission thus plays a central role in this pathway. In particular, the “vagal inflammatory reflex” concept has been outlined in the literature over time. In detail, Tracey et al. showed in preclinical models of endotoxic shock that there is an immunomodulatory cholinergic pathway with a sensory component and a motor component. In damaged peripheral tissues, the production of cytokines and other inflammatory molecules activates the vagal afferent pathway (sensory component) as a priming of the activation of the motor component of the pathway with the release of acetylcholine in the reticuloendothelial system (in the hepatic, cardiac, splenic and gastrointestinal context) and inhibition of the production and diffusion of molecules such as TNF, IL-1 and other cytokines. These considerations relate to the activity of the melanocortin system, as after observing the positive effects of melanocortins in the prevention of haemorrhagic shock, sepsis, myocardial ischaemia and stroke, the ability of melanocortins to activate the former cholinergic pathways via MC4R was highlighted [47,48][32][33]. It has also been observed that selective MC4R agonists can increase IL-6 and IL-11 levels at the glial level. These interleukins orient astrocytic macrophages towards an anti-inflammatory phenotype [49][34].3.5. MC5R
This receptor has been mainly associated with immune-mediated inflammatory modulation and induction of the JAK2-mediated pathway [25][13]. MC5R has been implicated in ocular immunity, although its role in inflammatory responses is not fully elucidated. However, selective MC5R activation showed anti-inflammatory actions in diabetic retinopathy, both in vitro and in vivo [33[22][23],34], with a specific antiangiogenic activity in human retinal pigment epithelial cells [60][35].4. The Role of the Melanocortin System in IBD
4.1. MC1R Mediates and Improves Intestinal Inflammation in Major Models of Experimental Colitis, and Some of Its Agonists Are Being Carefully Studied as Potential Therapeutic Agents in IBD
MC1R, and the pathways it activates, has been mainly studied in experimental colitis models showing how MC1R signalling pathways impact parameters related to colitis disease activity. The gene for MC1R is located on chromosome 16, the same chromosome where one of the genetic susceptibilities to CD has been identified (i.e., that nucleotide-binding oligomerisation domain containing 2/caspase recruitment domain-containing protein 15, also known as NOD2/CARD15) [81,82][36][37]. Among the first studies, Maaser et al. [64][38], in 2006, induced dextran sodium sulphate (DSS)-induced experimental colitis in a group of C57BL/6 mice with a frameshift mutation in the coding sequence for MC1R (MC1Re/e). The authors demonstrated how MC1Re/e and, thus, an impairment in MC1R-mediated signalling resulted in a worse course of DSS-induced experimental colitis in mutated mice than in wild-type (WT) mice. In detail, histologically, after DSS treatment, MC1Re/e mice showed more severe epithelial damage (with more ulcerations and a more pronounced inflammatory cell infiltrate) than MC1R-WT mice. Later, Kannengiesser et al. [66][39] assayed the anti-inflammatory action of a melanocortin-derived tripeptide, i.e., α-MSH(11-14), also called KPV (i.e., Lys-Pro-Val) or 5-phenyl-2-keto-valeric acid [83][40], in two models of experimental murine colitis: that induced by DSS and that of CD45RBhi mice transfer. This KPV peptide finds its anti-inflammatory rationale in that α-MSH exerts anti-inflammatory actions via the three amino acids 11–14 KPV located at the 3-terminal end [84][41]. The authors also examined the therapeutic potential of KPV in a third model of DSS-induced colitis in MC1Re/e mice. KPV improved DSS colitis and CD45RBhi mice transfer with an early weight regain compared to control animals and histological findings (e.g., leucitic infiltrate, submucosal oedema, crypt hyperplasia). Finally, an interesting finding was a very different behaviour of KPV in MC1Re/e mice. The therapeutic potential of KPV in murine DSS-induced experimental colitis was also confirmed in another study in which KPV was administered in nanoparticles [70,85][42][43]. It has, finally, been hypothesised that KPV is internalised within intestinal cells via a transporter peptide (i.e., PepT1) [86][44]. PepT1 is an H+-coupled di/tripeptide transporter localised at the apical membrane of intestinal epithelial cells [87][45]. It can transport H+ ions as well as peptides. Its expression is mainly localised at the small intestine level (as well as in the kidney and bile ducts) [87,88,89][45][46][47]. PepT1 does not have constitutively high levels of expression at the colonic level but may increase if inflammatory stimuli are chronically present at the colonic level [90][48]. Subsequent studies focused on two other MC1R receptor agonists (i.e., PL-8177 and PL-8331). In detail, Spana et al. [71][49] evaluated the in vitro anti-inflammatory action of PL-8177 and PL-8331 by assessing a reduction in TNF production (by setting ACTH and α-MSH as positive controls) in whole human blood previously stimulated with the bacterial lipopolysaccharide similarly to positive controls. More recently, the anti-inflammatory action of PL-8177 was also confirmed in another model of DNBS- and DSS-induced colitis in mice [73][50]. Specifically, however, the authors also evaluated the pharmacokinetics of PL-8177 after a single oral administration of 70 µg by marking it with 14C in dogs, showing a higher concentration in the colic than in the upper gastrointestinal tract.4.2. MC2R Is Involved in the Interaction between UVA and UVB and Murine DSS-Induced Colitis
Hiramoto et al. [69][51] studied the effect of UVA and UVB on mouse DSS-induced colitis concomitantly (i.e., the mouse was treated daily for five days with both DSS and UVA or UVB). The authors examined how UVA resulted in improvement of the typical symptoms while UVB, on the contrary, significantly worsened the course of DSS-induced colitis. However, the authors also evaluated the expression of MC2R in the colon, which was increased in UVB-treated and not UVA-treated mice. UVB treatment was associated with increased blood levels of ACTH, corticotropin-releasing hormone, urocortin-2, IL-6, IL-18 and histamine while, in contrast, β-endorphin levels were lower in UVA-treated mice. In UVA mice, blood levels of TNF and histamine were also reduced. In addition, the authors observed an improvement in UVB-induced DSS-induced colitis by administering an MC2R inhibitor (which appears to have receptor affinity for ACTH).4.3. Colic Expression of MC3R and MC4R Appears to Differ According to Disease Activity in IBD: Initial Experience and Scarce Evidence
Indeed, despite the more central localisation of MC4R, its role and peripheral localisations are increasingly emerging. Panaro et al. [93][52] showed how MC4R is expressed in different portions of the gastrointestinal tract, such as the stomach, small intestine and descending colon. Several cytotypes, such as small intestine cells positive for cholecystokinin, gastric inhibitory peptide, and glucagon-like peptide 1 of mice, showed high expression of the mRNA coding for this receptor. By stimulating it with α-MSH administration, its functions seem related to a paracrine inhibition of electrolyte secretion.
4.4. Combining Recombinant Bacteria and α-MSH as a Strategy in Experimental Colitis
α-MSH has also been studied as a therapeutic agent for experimental colitis by using an intestinal bacterium as the vehicle that delivered it to the colon (Figure 1). Specifically, Wei et al. [94][53] transformed Bifidobacterium longum strains with a specific plasmid (i.e., pBDMSH) to be α-MSH-expressing [68][54]. The authors then evaluated this recombinant bacterium in DSS-induced murine colitis (after testing the bioactivity of the modified bacterial strain in HT-29 cell models subjected to the inflammatory action of the bacterial lipopolysaccharide). This delivery system of α-MSH (administered orally to mice) was able to improve DSS-induced colitis by regulating the neutrophil infiltrate (as evidenced by modulation of myeloperoxidase) as well as by intervening in the cytokine imbalance by reducing the levels of TNF and IL-1β and IL-6 and increasing the levels of the anti-inflammatory cytokine IL-10.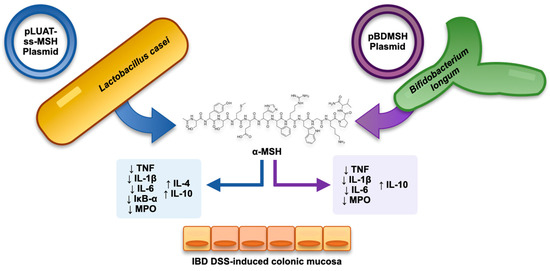
4.5. Exploiting the Similarity with KPV Tripeptide of the C-Terminal End of α-MSH: The Anti-Inflammatory Role of KPV-like Tripeptide KdPT
4.5. Exploiting the Similarity with KPV Tripeptide of the C-Terminal End of α-MSH: The Anti-Inflammatory Role of KPV-like Tripeptide KdPT
The KPV peptide, as already noted, has been the object of numerous studies conducted in the context of experimental colitis. Bettenworth et al. [95][55] studied a tripeptide structurally similar to KPV (i.e., KdPT, Figure 2) in the context of DSS-induced and IL-10-disrupted mice treated with piroxicam experimental colitis, showing promising potential in attenuating them. In detail, they showed a mechanism by which KdPT reinforced the tight junction between colonic cells. In addition, this peptide did not influence melanogenesis in vitro despite its molecular similarity with KPV.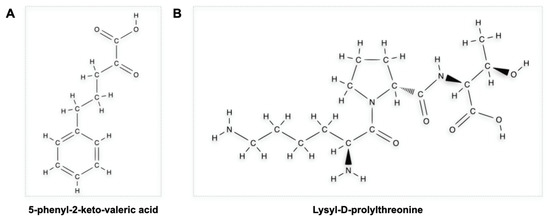
4.6. Not Only α-MSH: What Potential of β-MSH?
4.7. Prospects
5. Conclusions
In conclusion, several melanocortin family members seem interesting and promising when applied to the IBD field. It has been observed that melanocortins certainly have a role in modulating inflammatory processes, and their means of impacting the activity and pathogenesis of experimental IBD have also been shown. There is a clear need for studies evaluating the efficacy and safety of the use of melanocortin metabolites in the therapeutic management of IBD in the clinical setting.References
- Catania, A. The Melanocortin System in Leukocyte Biology. J. Leukoc. Biol. 2007, 81, 383–392.
- Holder, J.R.; Haskell-Luevano, C. Melanocortin Ligands: 30 Years of Structure-Activity Relationship (SAR) Studies. Med. Res. Rev. 2004, 24, 325–356.
- Yang, Y.; Harmon, C.M. Molecular Signatures of Human Melanocortin Receptors for Ligand Binding and Signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2436–2447.
- Videira, I.F.d.S.; Moura, D.F.L.; Magina, S. Mechanisms Regulating Melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83.
- Lam, C.; Getting, S. Melanocortin Receptor Type 3 as a Potential Target for Anti-Inflammatory Therapy. CDTIA 2004, 3, 311–315.
- Kawai, T.; Akira, S. Signaling to NF-KappaB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469.
- Lawrence, T. The Nuclear Factor NF-KappaB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651.
- Thoma, A.; Lightfoot, A.P. NF-KB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 267–279.
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB System. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241.
- Cheng, Z.; Taylor, B.; Ourthiague, D.R.; Hoffmann, A. Distinct Single-Cell Signaling Characteristics Are Conferred by the MyD88 and TRIF Pathways during TLR4 Activation. Sci. Signal. 2015, 8, ra69.
- Cui, H.-S.; Hayasaka, S.; Zhang, X.-Y.; Chi, Z.-L.; Hayasaka, Y. Effect of Alpha-Melanocyte-Stimulating Hormone on Interleukin 8 and Monocyte Chemotactic Protein 1 Expression in a Human Retinal Pigment Epithelial Cell Line. Ophthalmic Res. 2005, 37, 279–288.
- Haycock, J.W.; Wagner, M.; Morandini, R.; Ghanem, G.; Rennie, I.G.; Mac Neil, S. Alpha-Melanocyte-Stimulating Hormone Inhibits NF-KappaB Activation in Human Melanocytes and Melanoma Cells. J. Investig. Dermatol. 1999, 113, 560–566.
- Buggy, J.J. Binding of Alpha-Melanocyte-Stimulating Hormone to Its G-Protein-Coupled Receptor on B-Lymphocytes Activates the Jak/STAT Pathway. Biochem. J. 1998, 331 Pt 1, 211–216.
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Schölmerich, J.; Gross, V. Nuclear Factor KappaB Is Activated in Macrophages and Epithelial Cells of Inflamed Intestinal Mucosa. Gastroenterology 1998, 115, 357–369.
- Pallone, F.; Monteleone, G. Mechanisms of Tissue Damage in Inflammatory Bowel Disease. Curr. Opin. Gastroenterol. 2001, 17, 307–312.
- Chang, S.H.; Jung, E.J.; Lim, D.G.; Park, Y.H.; Wee, Y.M.; Kim, J.H.; Kim, Y.H.; Choi, M.Y.; Koo, S.K.; Choi, K.D.; et al. Anti-Inflammatory Action of Alpha-Melanocyte Stimulating Hormone (Alpha-MSH) in Anti-CD3/CD28-Mediated Spleen and CD4(+)CD25(-) T Cells and a Partial Participation of IL-10. Immunol. Lett. 2008, 118, 44–48.
- Nishida, T.; Taylor, A.W. Specific Aqueous Humor Factors Induce Activation of Regulatory T Cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2268–2274.
- Taylor, A.W.; Streilein, J.W.; Cousins, S.W. Alpha-Melanocyte-Stimulating Hormone Suppresses Antigen-Stimulated T Cell Production of Gamma-Interferon. Neuroimmunomodulation 1994, 1, 188–194.
- Auriemma, M.; Brzoska, T.; Klenner, L.; Kupas, V.; Goerge, T.; Voskort, M.; Zhao, Z.; Sparwasser, T.; Luger, T.A.; Loser, K. α-MSH-Stimulated Tolerogenic Dendritic Cells Induce Functional Regulatory T Cells and Ameliorate Ongoing Skin Inflammation. J. Investig. Dermatol. 2012, 132, 1814–1824.
- Saporiti, F.; Piacentini, L.; Alfieri, V.; Bono, E.; Ferrari, F.; Chiesa, M.; Colombo, G.I. Melanocortin-1 Receptor Positively Regulates Human Artery Endothelial Cell Migration. Cell. Physiol. Biochem. 2019, 52, 1339–1360.
- Seaton, M.E.; Parent, B.A.; Sood, R.F.; Wurfel, M.M.; Muffley, L.A.; O’Keefe, G.E.; Gibran, N.S. Melanocortin-1 Receptor Polymorphisms and the Risk of Complicated Sepsis After Trauma: A Candidate Gene Association Study. Shock 2017, 47, 79–85.
- Rossi, S.; Maisto, R.; Gesualdo, C.; Trotta, M.C.; Ferraraccio, F.; Kaneva, M.K.; Getting, S.J.; Surace, E.; Testa, F.; Simonelli, F.; et al. Corrigendum to “Activation of Melanocortin Receptors MC1 and MC5 Attenuates Retinal Damage in Experimental Diabetic Retinopathy”. Mediat. Inflamm. 2021, 2021, 1–2.
- Maisto, R.; Gesualdo, C.; Trotta, M.C.; Grieco, P.; Testa, F.; Simonelli, F.; Barcia, J.M.; D’Amico, M.; Di Filippo, C.; Rossi, S. Melanocortin Receptor Agonists MCR1-5 Protect Photoreceptors from High-Glucose Damage and Restore Antioxidant Enzymes in Primary Retinal Cell Culture. J. Cell. Mol. Med. 2017, 21, 968–974.
- Andersen, M.; Nagaev, I.; Meyer, M.K.; Nagaeva, O.; Wikberg, J.; Mincheva-Nilsson, L.; Andersen, G.N. Melanocortin 2, 3 and 4 Receptor Gene Expressions Are Downregulated in CD8+ T Cytotoxic Lymphocytes and CD19+ B Lymphocytes in Rheumatoid Arthritis Responding to TNF-α Inhibition. Scand. J. Immunol. 2017, 86, 31–39.
- Getting, S.J.; Perretti, M. MC3-R as a Novel Target for Antiinflammatory Therapy. Drug News Perspect. 2000, 13, 19–27.
- Getting, S.J.; Gibbs, L.; Clark, A.J.; Flower, R.J.; Perretti, M. POMC Gene-Derived Peptides Activate Melanocortin Type 3 Receptor on Murine Macrophages, Suppress Cytokine Release, and Inhibit Neutrophil Migration in Acute Experimental Inflammation. J. Immunol. 1999, 162, 7446–7453.
- Getting, S.J.; Di Filippo, C.; Christian, H.C.; Lam, C.W.; Rossi, F.; D’Amico, M.; Perretti, M. MC-3 Receptor and the Inflammatory Mechanisms Activated in Acute Myocardial Infarct. J. Leukoc. Biol. 2004, 76, 845–853.
- Catania, A. Neuroprotective Actions of Melanocortins: A Therapeutic Opportunity. Trends Neurosci. 2008, 31, 353–360.
- Mountjoy, K.G.; Mortrud, M.T.; Low, M.J.; Simerly, R.B.; Cone, R.D. Localization of the Melanocortin-4 Receptor (MC4-R) in Neuroendocrine and Autonomic Control Circuits in the Brain. Mol. Endocrinol. 1994, 8, 1298–1308.
- Tracey, K.J. Physiology and Immunology of the Cholinergic Antiinflammatory Pathway. J. Clin. Investig. 2007, 117, 289–296.
- Zila, I.; Mokra, D.; Kopincova, J.; Kolomaznik, M.; Javorka, M.; Calkovska, A. Vagal-Immune Interactions Involved in Cholinergic Anti-Inflammatory Pathway. Physiol. Res. 2017, 66, S139–S145.
- Giuliani, D.; Ottani, A.; Altavilla, D.; Bazzani, C.; Squadrito, F.; Guarini, S. Melanocortins and the Cholinergic Anti-Inflammatory Pathway. Adv. Exp. Med. Biol. 2010, 681, 71–87.
- Tracey, K.J. The Inflammatory Reflex. Nature 2002, 420, 853–859.
- Kamermans, A.; Verhoeven, T.; van Het Hof, B.; Koning, J.J.; Borghuis, L.; Witte, M.; van Horssen, J.; de Vries, H.E.; Rijnsburger, M. Setmelanotide, a Novel, Selective Melanocortin Receptor-4 Agonist Exerts Anti-Inflammatory Actions in Astrocytes and Promotes an Anti-Inflammatory Macrophage Phenotype. Front. Immunol. 2019, 10, 2312.
- Maisto, R.; Oltra, M.; Vidal-Gil, L.; Martínez-Gil, N.; Sancho-Pellúz, J.; Filippo, C.D.; Rossi, S.; D’Amico, M.; Barcia, J.M.; Romero, F.J. ARPE-19-Derived VEGF-Containing Exosomes Promote Neovascularization in HUVEC: The Role of the Melanocortin Receptor 5. Cell Cycle 2019, 18, 413–424.
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 Leucine-Rich Repeat Variants with Susceptibility to Crohn’s Disease. Nature 2001, 411, 599–603.
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A Frameshift Mutation in NOD2 Associated with Susceptibility to Crohn’s Disease. Nature 2001, 411, 603–606.
- Maaser, C. Crucial Role of the Melanocortin Receptor MC1R in Experimental Colitis. Gut 2006, 55, 1415–1422.
- Kannengiesser, K.; Maaser, C.; Heidemann, J.; Luegering, A.; Ross, M.; Brzoska, T.; Bohm, M.; Luger, T.A.; Domschke, W.; Kucharzik, T. Melanocortin-Derived Tripeptide KPV Has Anti-Inflammatory Potential in Murine Models of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2008, 14, 324–331.
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380.
- Luger, T.A.; Scholzen, T.E.; Brzoska, T.; Böhm, M. New Insights into the Functions of Alpha-MSH and Related Peptides in the Immune System. Ann. N. Y. Acad. Sci. 2003, 994, 133–140.
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis. Mol. Ther. 2017, 25, 1628–1640.
- Laroui, H.; Dalmasso, G.; Nguyen, H.T.T.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Drug-Loaded Nanoparticles Targeted to the Colon with Polysaccharide Hydrogel Reduce Colitis in a Mouse Model. Gastroenterology 2010, 138, 843–853.
- Dalmasso, G.; Charrier-Hisamuddin, L.; Nguyen, H.T.T.; Yan, Y.; Sitaraman, S.; Merlin, D. PepT1-Mediated Tripeptide KPV Uptake Reduces Intestinal Inflammation. Gastroenterology 2008, 134, 166–178.
- Steel, A.; Nussberger, S.; Romero, M.F.; Boron, W.F.; Boyd, C.A.; Hediger, M.A. Stoichiometry and PH Dependence of the Rabbit Proton-Dependent Oligopeptide Transporter PepT1. J. Physiol. 1997, 498 Pt 3, 563–569.
- Shen, H.; Smith, D.E.; Yang, T.; Huang, Y.G.; Schnermann, J.B.; Brosius, F.C. Localization of PEPT1 and PEPT2 Proton-Coupled Oligopeptide Transporter MRNA and Protein in Rat Kidney. Am. J. Physiol. 1999, 276, F658–F665.
- Daniel, H. Molecular and Integrative Physiology of Intestinal Peptide Transport. Annu. Rev. Physiol. 2004, 66, 361–384.
- Merlin, D.; Si-Tahar, M.; Sitaraman, S.V.; Eastburn, K.; Williams, I.; Liu, X.; Hediger, M.A.; Madara, J.L. Colonic Epithelial HPepT1 Expression Occurs in Inflammatory Bowel Disease: Transport of Bacterial Peptides Influences Expression of MHC Class 1 Molecules. Gastroenterology 2001, 120, 1666–1679.
- Spana, C.; Taylor, A.W.; Yee, D.G.; Makhlina, M.; Yang, W.; Dodd, J. Probing the Role of Melanocortin Type 1 Receptor Agonists in Diverse Immunological Diseases. Front. Pharmacol. 2019, 9, 1535.
- Dodd, J.; Jordan, R.; Makhlina, M.; Barnett, K.; Roffel, A.; Spana, C.; Obr, A.; Dhingra, P.; Kayne, P.S. A Novel Oral Formulation of the Melanocortin-1 Receptor Agonist PL8177 Resolves Inflammation in Preclinical Studies of Inflammatory Bowel Disease and Is Gut Restricted in Rats, Dogs, and Humans. Front. Immunol. 2023, 14, 1083333.
- Hiramoto, K.; Yamate, Y.; Sato, E.F. The Effects of Ultraviolet Eye Irradiation on Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. Photochem. Photobiol. 2016, 92, 728–734.
- Panaro, B.L.; Tough, I.R.; Engelstoft, M.S.; Matthews, R.T.; Digby, G.J.; Møller, C.L.; Svendsen, B.; Gribble, F.; Reimann, F.; Holst, J.J.; et al. The Melanocortin-4 Receptor Is Expressed in Enteroendocrine L Cells and Regulates the Release of Peptide YY and Glucagon-like Peptide 1 In Vivo. Cell Metab. 2014, 20, 1018–1029.
- Wei, P.; Yang, Y.; Liu, Z.; Huang, J.; Gong, Y.; Sun, H. Oral Bifidobacterium Longum Expressing Alpha-Melanocyte-Stimulating Hormone to Fight Experimental Colitis. Drug Deliv. 2016, 23, 2058–2064.
- Wei, P.; Yang, Y.; Ding, Q.; Li, X.; Sun, H.; Liu, Z.; Huang, J.; Gong, Y. Oral Delivery of Bifidobacterium Longum Expressing α-Melanocyte-Stimulating Hormone to Combat Ulcerative Colitis. J. Med. Microbiol. 2016, 65, 160–168.
- Bettenworth, D.; Buyse, M.; Böhm, M.; Mennigen, R.; Czorniak, I.; Kannengiesser, K.; Brzoska, T.; Luger, T.A.; Kucharzik, T.; Domschke, W.; et al. The Tripeptide KdPT Protects from Intestinal Inflammation and Maintains Intestinal Barrier Function. Am. J. Pathol. 2011, 179, 1230–1242.
- Spencer, J.D.; Schallreuter, K.U. Regulation of Pigmentation in Human Epidermal Melanocytes by Functional High-Affinity Beta-Melanocyte-Stimulating Hormone/Melanocortin-4 Receptor Signaling. Endocrinology 2009, 150, 1250–1258.
- Luger, T.A.; Scholzen, T.; Brzoska, T.; Becher, E.; Slominski, A.; Paus, R. Cutaneous Immunomodulation and Coordination of Skin Stress Responses by Alpha-Melanocyte-Stimulating Hormone. Ann. N. Y. Acad. Sci. 1998, 840, 381–394.
- Bradamante, M.; Turčić, P.; Stambuk, N.; Konjevoda, P.; Aralica, G.; Alerić, I.; Kozmar, A. Cytoprotective Effects of β-Melanocortin in the Rat Gastrointestinal Tract. Molecules 2012, 17, 11680–11692.
- Yeo, G.S.H.; Chao, D.H.M.; Siegert, A.-M.; Koerperich, Z.M.; Ericson, M.D.; Simonds, S.E.; Larson, C.M.; Luquet, S.; Clarke, I.; Sharma, S.; et al. The Melanocortin Pathway and Energy Homeostasis: From Discovery to Obesity Therapy. Mol. Metab. 2021, 48, 101206.
