Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Zhenyang Li.
Heterogeneous nuclear ribonucleoproteins (hnRNPs) are a superfamily of RNA-binding proteins consisting of more than 20 members. These proteins play a crucial role in various biological processes by regulating RNA splicing, transcription, and translation through their binding to RNA.
- hnRNPs
- alternative splicing
- muscle development
- muscle disorders
1. Introduction
Various post-transcriptional modifications are required for the maturation of mRNA in eukaryotic cells. These modifications include the addition of 7-methylguanosine (m7G) at the 5′ end, the formation of a polyadenylic acid tail at the 3′ end, and RNA splicing. Alternative splicing allows for the production of different mature mRNAs from a single pre-mRNA molecule. Heterogeneous nuclear ribonucleoproteins (hnRNPs) are a superfamily of RNA-binding proteins that play a key role in regulating the alternative splicing of pre-mRNA [1].
2. Overview of hnRNPs
2.1. Composition of hnRNPs
Heterogeneous nuclear ribonucleoproteins (hnRNPs) are a class of RNA-binding proteins (RBPs). Through immunopurification and two-dimensional gel electrophoresis, 42 kinds of hnRNPs have been identified in Hela cells. These hnRNPs consist of more than 20 major hnRNPs that exhibit relatively higher abundance, along with other hnRNPs [2,3][2][3]. The main hnRNPs have a molecular weight ranging from 34 kDa to 120 kDa and are named hnRNP A1 to hnRNP U [4,5,6,7][4][5][6][7] based on their molecular weight and structural and functional characteristics. Due to the strong association between hnRNP A1, A2/B1, B2, C1, and C2 and hnRNA, they are referred to as “core proteins” [8].2.2. hnRNPs: RNA-Binding Domains and Structural Insights
The analysis of cDNA sequences has revealed that hnRNPs possess a modular structure comprising at least one RNA-binding motif and auxiliary domains [9,10][9][10] (Figure 1). As recent investigations into the structure of hnRNPs have delved into greater detail, it has been confirmed that hnRNPs consist of three distinct RNA-binding motifs: the RNA recognition motif (RRM) [11[11][12],12], the RNA-binding domain consisting of Arg-Gly-Gly repeats (RGG domain) [13], and the K-homology domain (KH domain) [14].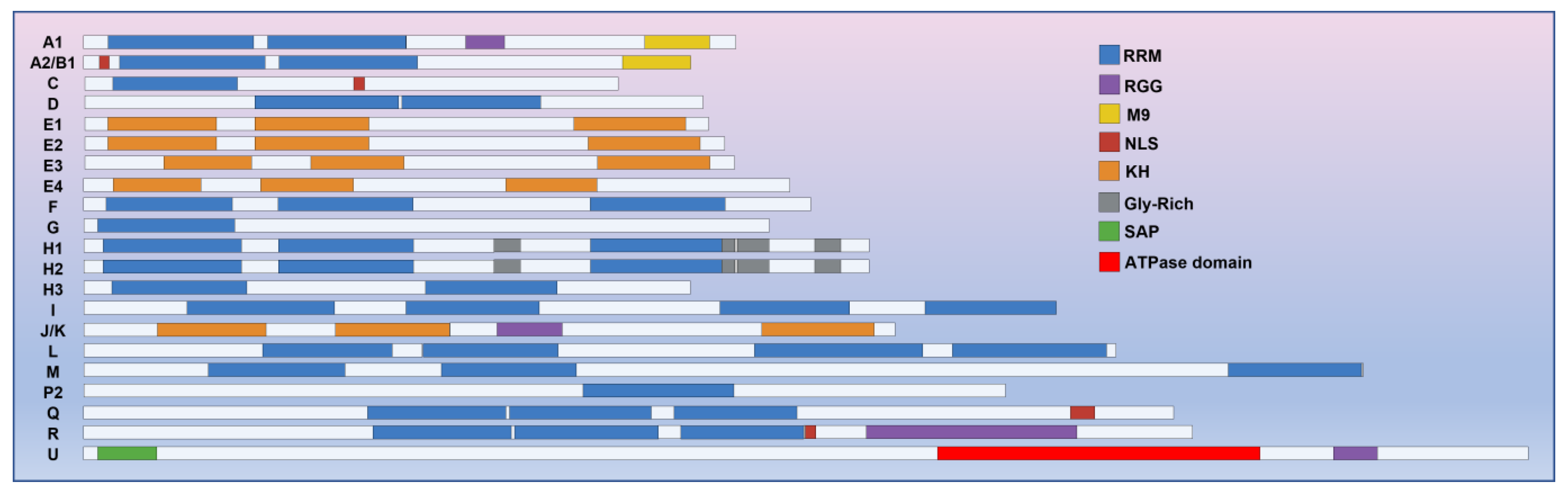
Figure 1. hnRNPs consist of three types of RNA binding motifs: RRM, RGG, and KH domains. The RRM motif is the most prevalent among these motifs in hnRNPs. Apart from RNA-binding motifs, certain hnRNPs may also possess auxiliary domains, namely the M9, NLS, SAP, and ATPase domains. These auxiliary domains play crucial roles in various processes such as nucleocytoplasmic shuttling, DNA binding, and the oligomerization of hnRNPs with caRNAs.
2.3. Functions of hnRNPs
Due to the diverse types and complex structural domains of hnRNPs, they can perform a variety of functions in cells. These functions include processing pre-mRNA into mature mRNA and serving as trans-acting factors that regulate gene expression. hnRNPs also participate in the processing of pre-mRNA alongside other RNPs, playing a crucial role in regulating mRNA transport, localization, translation, and stability (Figure 2). As a result, hnRNPs play a key role in various biological processes within cells. Contemporary research on hnRNPs primarily focuses on their involvement in muscle and neurodegenerative diseases (such as amyotrophic lateral sclerosis) as well as their role in cancer progression [40,41,42][40][41][42].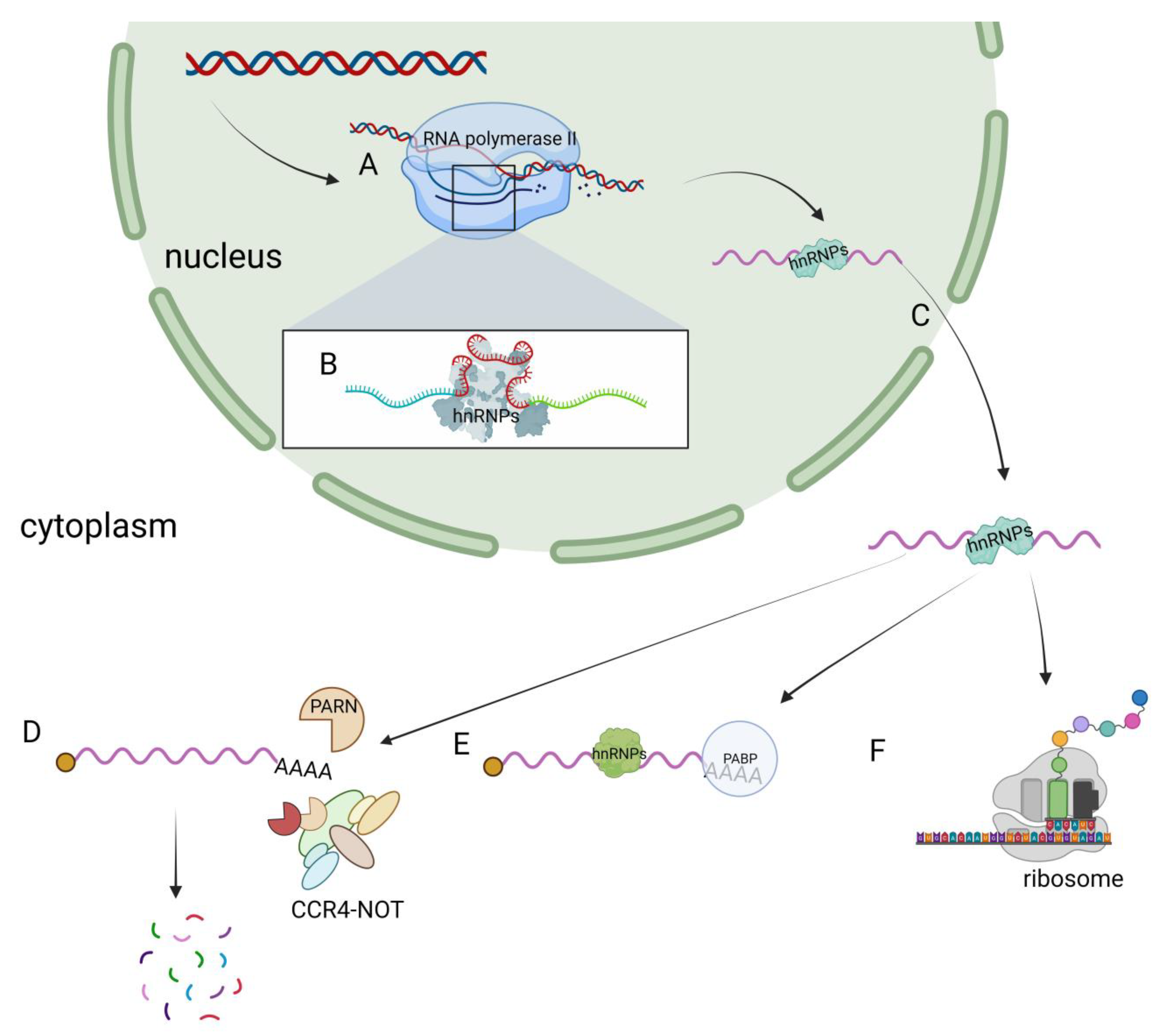
Figure 2. Functions of hnRNPs in cells. (A) hnRNPs have transcriptional regulatory functions. (B) hnRNPs regulate the alternative splicing of pre-mRNA. (C) The nucleocytoplasmic shuttling function of hnRNPs. (D) hnRNPs are involved in the degradation of mRNA. (E) hnRNPs participate in the regulation of mRNA stability. (F) hnRNPs regulate the translation process.
2.3.1. Regulation of Alternative Splicing
The pre-mRNA splicing process in cells relies on the interaction between splicing factors and splice sites. This mainly includes the following steps: (1) The U1 snRNP complex binds to the 5′ splice site [43,44][43][44]. (2) U2AF65 binds to the polypyrimidine site (Py-tract) [45]. (3) U2AF35 binds to the AG bases at the 3′ splice site. (4) The U2AF heterodimer binds to the 3′ splice site [46] (Figure 3). Most hnRNPs typically function as inhibitory splicing regulators, and their mechanism is as follows.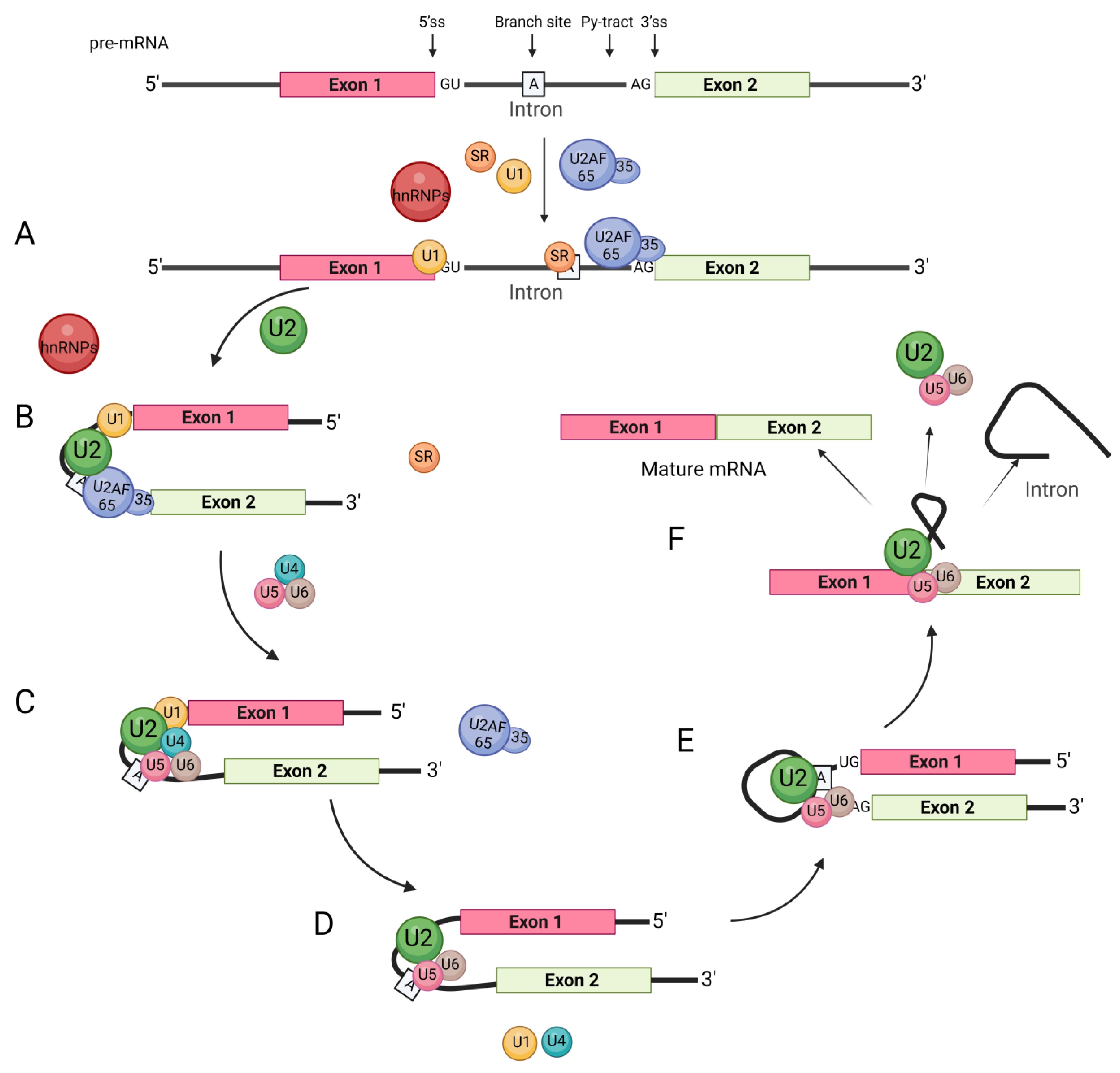
Figure 3. General splicing process. The regulation of RNA-binding proteins such as hnRNPs and SRs involves several steps. (A) The splicing factor U1 snRNP complex binds to the 5′ splice site, U2AF65 binds to the polypyrimidine site (Py-tract), and U2AF35 binds to the AG bases at the 3′ splice site. The U2AF heterodimer combines with the 3′ splicing site to recognize the intron splicing signal. (B) U2 snRNP binds to the branch site with the assistance of U2AF. (C) snRNP U4, U6, U5 join the complex, while U2AF dissociates from the complex. (D) U1 snRNP and U4 snRNP subsequently leave the complex through a series of conformational transitions. (E) The first transesterification reaction connects the 5′ss to the branch site and cleaves the RNA strand, forming a lariat structure. (F) In the second transesterification reaction, the exons are ligated to each other to form the mRNA, and the introns are released in a lariat structure.
-
Splice site identification and shelter: hnRNP proteins have the ability to bind to pre-mRNA splice sites, which can impact the formation of spliceosomes. This binding can mask or hinder the recognition of splice sites, leading to differential alternative splicing. Additionally, the binding of hnRNPs can competitively affect the binding of other splicing factors. For instance, hnRNP I’s RRM1 and RRM2 specifically bind to the polypyrimidine sequence of the fourth internal loop of the U1 snRNA stem loop, thereby inactivating the splicing complex A formed by U1 snRNP [47]. Studies have also shown that hnRNP I can target ITSN1 [48], β-tropomyosin [49], Fas [50], and alpha-actinin [51], competitively inhibiting the binding of U2AF65 and preventing the formation of the U2AF heterodimer complex by binding to the polypyrimidine sequence of pre-mRNA. hnRNP A1 interacts with the 3′ splice site of MAPT exon 10 and facilitates the exclusion of exon 10 [52]. Similarly, hnRNP L can bind to pre-mRNA polypyrimidine sequences, competitively inhibiting the binding of U2AF65 to RNA [53]. Furthermore, hnRNP H1/H2 counteracts the activation of the 3′ splice site [54] (Figure 4B).
-
Splicing inhibition: hnRNPs can impact RNA structure by interacting with pre-mRNA, resulting in the exclusion of specific exons. This inhibitory effect primarily operates by altering the structure of the splice site. For instance, the RRM3 and RRM4 domains of hnRNP I bind to the polypyrimidine sequence (e.g., CUCUCU) near the pre-mRNA exon, forming an RNA ring structure that hinders the binding of other splicing factors and the formation of splicing complexes [51,55][51][55] (Figure 4C).
-
Competitive splicing inhibition: In certain cases, hnRNP proteins and other RNA-binding proteins may competitively bind to the same pre-mRNA, which can impact the inclusion or exclusion of specific exons. The majority of competitive splicing occurs between hnRNPs and SRs. For instance, hnRNP H1 can compete with SRSF3 for binding to PRMT5 pre-mRNA, thereby inhibiting the exclusion of PRMT5 exon 3 by SRSF3 [56]. Additionally, hnRNPA1 can competitively bind to the G-rich sequence downstream of β-tropomyosin exon 6B, along with ASF/SF2, leading to the inhibition of exon 6B exclusion. Simultaneously, hnRNP A1 and ASF/SF2 competitively bind to the 5′ splice site of C175G pre-mRNA (C175G is a synthetic 533 nt pre-mRNA sequence that is frequently employed as a standard model for investigating 5′ splice sites). hnRNP A1 competitively inhibits the binding of U1 snRNP to the 5′ splice site of C175G pre-mRNA, while ASF/SF2 enhances the binding of U1 snRNP to the 5′ splice site of C175G pre-mRNA [57] (Figure 4D).
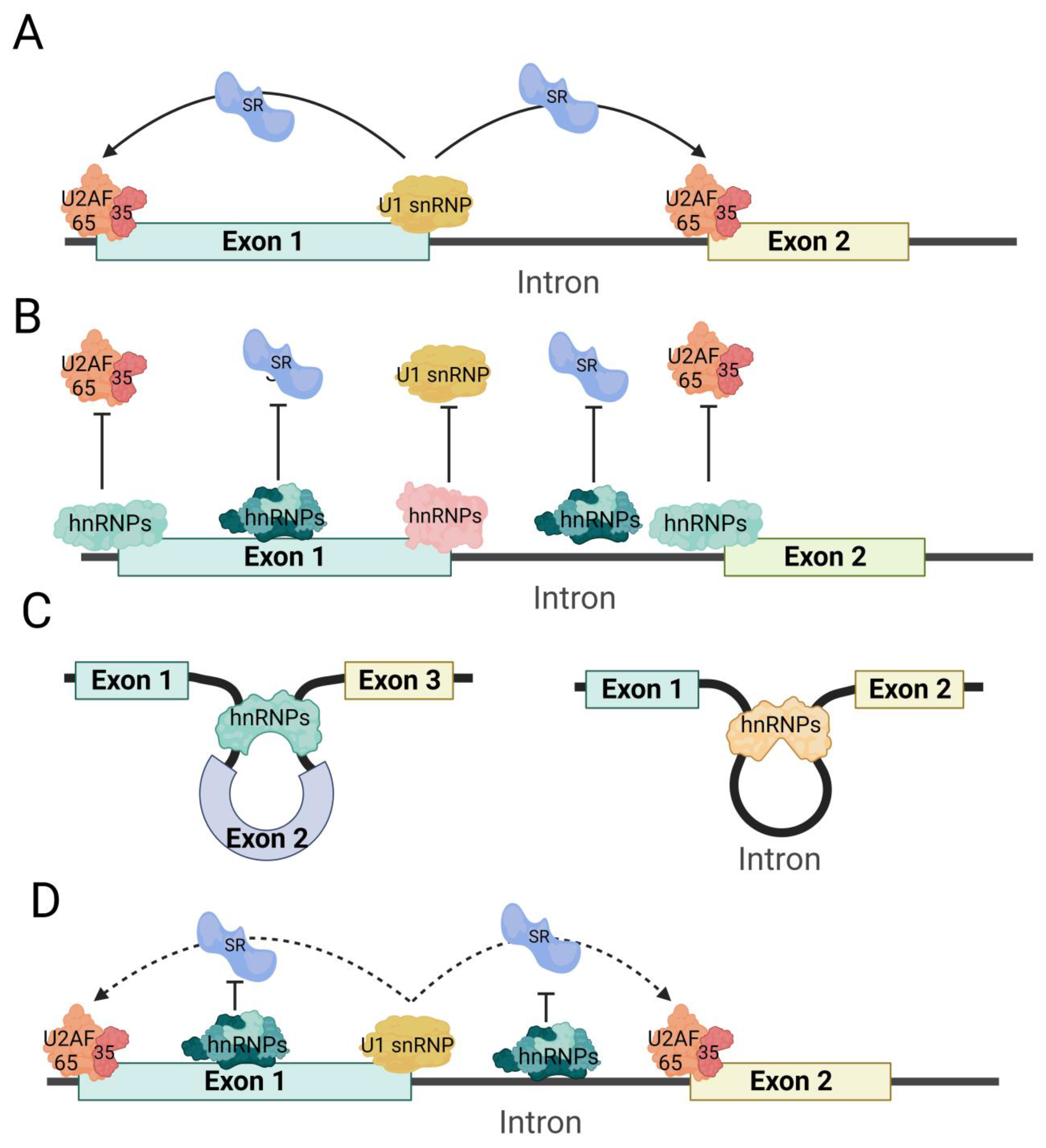
Figure 4. Schematic diagram of hnRNPs regulating variable splicing. The process of exon excision (left) and intron excision (right) shown in panel (A) relates to when hnRNPs are not involved in variable splicing. Panel (B) demonstrates how hnRNPs prevent splicing factors such as U2AF heterodimer and U1 snRNPs from binding to pre-mRNA. This prevention is achieved through the recognition of splicing sites and masking effects. In panel (C), hnRNPs are shown to promote exon (left) and intron (right) retention by binding to pre-mRNA, forming a special pre-mRNA loop structure. This structure prevents splicing factors from recognizing splice sites on pre-mRNA. Finally, in panel (D), hnRNPs bind to SRs and hinder the promotion of splicing by SRs.
2.3.2. Regulation of mRNA Stability
hnRNPs play a role in regulating mRNA stability through various mechanisms, including the poly(A) tail, AU-rich elements (AREs), and the 3′UTR. For instance, hnRNP H1 and hnRNP F can enhance the stability of APP mRNA by binding to it through cytoplasmic shuttling mediated by the gly Rich motif, thereby increasing its half-life [63]. Additionally, hnRNP F has been found to regulate the TTP/BRF-mediated degradation of ARE-mRNAs [64]. The RRM2 domain of hnRNP A2/B1 acts as an m6A reader [65], recognizing the N6-methyladenosine (m6A) site on TCF7L2 mRNA to stabilize the poly(A) tail and maintain its mRNA stability. Moreover, hnRNP A2/B1 and hnRNP A1 play a role in the regulation of mRNA deadenylation via the CCR4-NOT deadenylation complex. They achieve this by binding to the UAASUUAU sequence present in the mRNA 3′UTR, thereby influencing the degradation of the transcript [66] (Figure 5B). Interestingly, hnRNP A2/B1 also binds to its own 3′UTR to regulate the ratio of nonsense-mediated RNA decay (NMD) (both for sensitive and insensitive types). When the levels of hnRNP A2/B1 protein increase, it combines with its own pre-mRNA 3′UTR, leading to alternative splicing and the production of a higher proportion of NMD-sensitive mRNAs. These NMD-sensitive mRNAs are subsequently degraded during translation [67]. This auto-regulatory mechanism demonstrates how splicing factors control their own expression levels, providing one possible explanation [68]. As an ARE-binding protein, hnRNP D has the ability to recognize and bind to various ARE-mRNA sequences by targeting uridine residues [69]. Subsequently, it recruits transporters such as eIF4G, PABP, Hsp70, Hsc70, and Hsp27 to form the signal transduction regulatory complex (ASTRC). ASTRC plays a crucial role in initiating the 3′ to 5′ deadenylation-dependent mRNA degradation pathway, thereby promoting mRNA degradation. Among the isoforms of hnRNP D, the specific mechanism of action involves AUF1 interacting with the AU-rich element (ARE) in mRNA, which serves as a recognition site for AUF1. While the mRNA is being translated, AUF1 interacts with the translation initiation factor eIF4G [70], causing AUF1 to be released from the ARE by the ribosome. This enables the ribosome to access the mRNA and initiate translation. Simultaneously, AUF1 forms a complex with the polyadenine nucleic acid-binding protein (PABP), which exposes the polyadenine nucleic acid tail and allows the mRNA to be degraded by nuclease. The p40 isoform is involved in the decay of ARE-mRNA, which regulates lymphokine mRNA stability [71] (Figure 5A). Additionally, studies have demonstrated [72] that the KH3 domain of hnRNP K can bind to the poly(C) site of LAPTM5 3′UTR, thereby enhancing the stability of its transcripts. Different hnRNPs exhibit variations in their regulation of mRNA stability. For instance, hnRNP D, K, I, and Q [73] can all bind to the 3′UTR of mPer3, but they differ in their impact on mPer3 stability. hnRNP K maintains the stability of mPer3, while hnRNP D and Q accelerate its degradation. hnRNP I, on the other hand, does not affect the stability of mPer3. Additionally, hnRNP A1 [74], C [75], U [76], and L [77] can bind to the 3′UTR of certain mRNAs and influence their stability, although the mechanism underlying this remains unclear.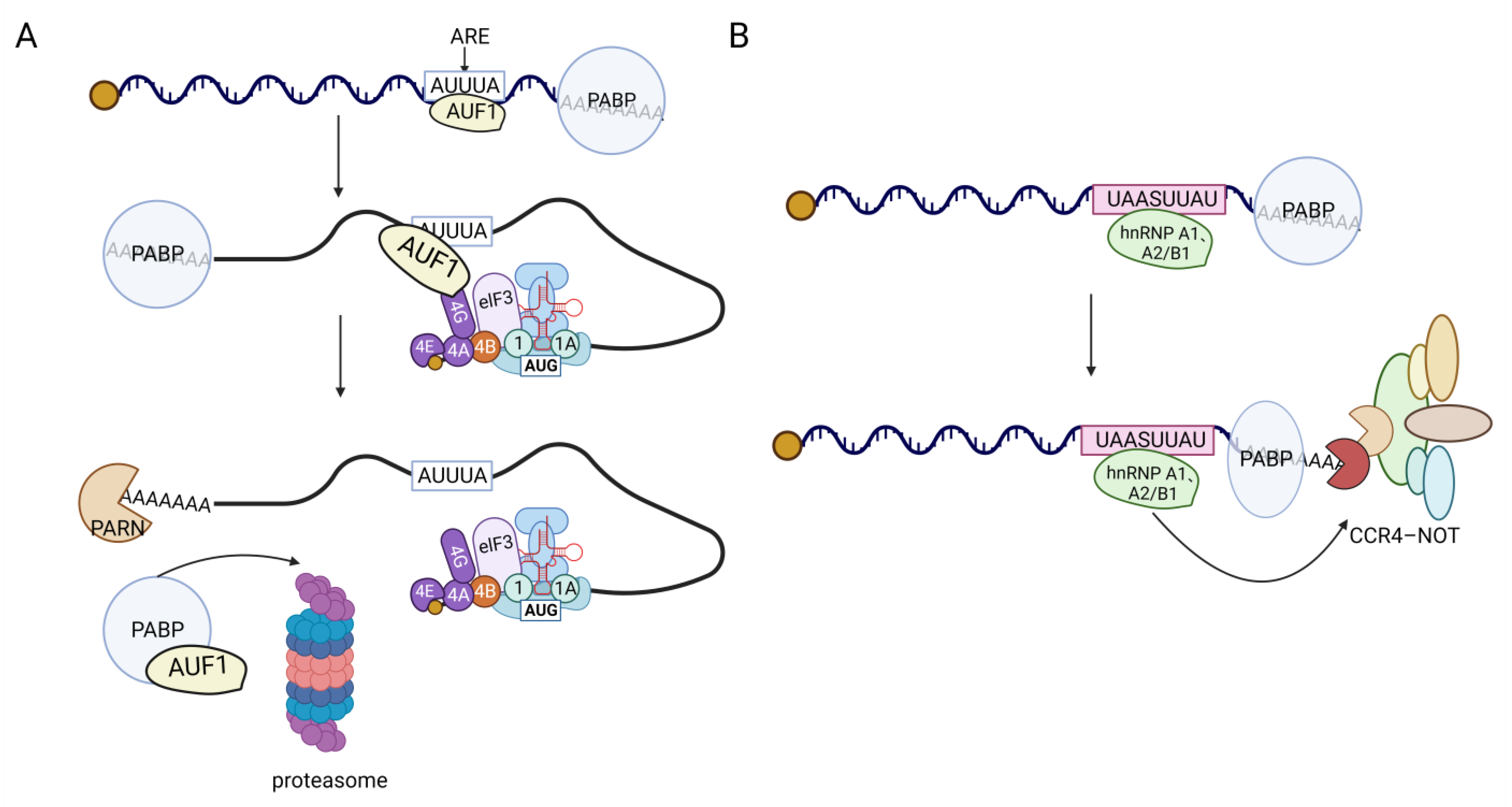
Figure 5. hnRNPs-mediated mRNA degradation mechanism. (A) In the translation process, eIF4G binds to AUF1, promoting the dissociation of AUF1 from the ARE sequence. The released AUF1 then binds to PABP, leading to the exposure of the polyadenine nucleic acid tail for mRNA degradation. Both AUF1 and PABP are subsequently degraded through the proteasome. (B) hnRNP A2/B1 and hnRNP A1 interact with the UAASUUAU sequence located in the 3′UTR of mRNA. This interaction plays a role in controlling mRNA deadenylation, which is carried out by the CCR4-NOT deadenylation complex. Ultimately, this process influences the degradation of the transcript.
2.3.3. Localization and Transport of mRNAs
RNA molecules transported within cells often contain specific cis-acting elements that are recognized by specific trans-acting factors in the cell. The interaction between these cis-acting elements and trans-acting factors, along with the involvement of molecular motors, enables RNA particles to actively transport on microtubules and actin filaments. The hnRNP AB and hnRNP A2 proteins contain RRM motifs that can bind to the cis-acting element RTS on the 3′UTR of mRNA [78]. This binding facilitates the transfer of mRNA from the nucleus to the cytoplasm. During this process, hnRNP AB may target specific transcripts by stabilizing RNA G4 quadruplexes near the transcript’s RTS. Several transcripts, including MBP, β-actin, Arc, BDNF, CAMKIIα, and Protamine 2, require hnRNP AB for their localization and transport [79,80,81,82,83][79][80][81][82][83]. The glycine-rich domain (GYR) of the hnRNP H/F protein interacts with Transportin 1 to facilitate nucleocytoplasmic shuttling and participate in the extranuclear transport of mRNA [84]. The M9 domain of hnRNP A1 is considered the main functional domain for nucleocytoplasmic shuttling, and its nuclear import does not rely on the classic NLS pathway [85].References
- Martinez-Contreras, R.; Cloutier, P.; Shkreta, L.; Fisette, J.; Revil, T.; Chabot, B. hnRNP proteins and splicing control. Adv. Exp. Med. Biol. 2007, 623, 123–147.
- Krecic, A.; Swanson, M. hnRNP complexes: Composition, structure, and function. Curr. Opin. Cell Biol. 1999, 11, 363–371.
- Singh, R.; Valcárcel, J. Building specificity with nonspecific RNA-binding proteins. Nat. Struct. Mol. Biol. 2005, 12, 645–653.
- Piñol-Roma, S.; Choi, Y.; Matunis, M.; Dreyfuss, G. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes Dev. 1988, 2, 215–227.
- Dreyfuss, G.; Philipson, L.; Mattaj, I.W. Ribonucleoprotein particles in cellular processes. J. Cell Biol. 1988, 106, 1419–1425.
- Dreyfuss, G.; Matunis, M.; Piñol-Roma, S.; Burd, C. hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 1993, 62, 289–321.
- Black, D. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003, 72, 291–336.
- Beyer, A.; Christensen, M.; Walker, B.; LeStourgeon, W. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell 1977, 11, 127–138.
- Chaudhury, A.; Chander, P.; Howe, P. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1′s multifunctional regulatory roles. RNA 2010, 16, 1449–1462.
- Dreyfuss, G.; Kim, V.; Kataoka, N. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 2002, 3, 195–205.
- Query, C.; Bentley, R.; Keene, J. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell 1989, 57, 89–101.
- Görlach, M.; Wittekind, M.; Beckman, R.; Mueller, L.; Dreyfuss, G. Interaction of the RNA-binding domain of the hnRNP C proteins with RNA. EMBO J. 1992, 11, 3289–3295.
- Kiledjian, M.; Dreyfuss, G. Primary structure and binding activity of the hnRNP U protein: Binding RNA through RGG box. EMBO J. 1992, 11, 2655–2664.
- Burd, C.; Dreyfuss, G. Conserved structures and diversity of functions of RNA-binding proteins. Science 1994, 265, 615–621.
- Bandziulis, R.J.; Swanson, M.S.; Dreyfuss, G. RNA-binding proteins as developmental regulators. Genes Dev. 1989, 3, 431–437.
- Nagai, K.; Oubridge, C.; Jessen, T.; Li, J.; Evans, P. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature 1990, 348, 515–520.
- Allain, F.; Gubser, C.; Howe, P.; Nagai, K.; Neuhaus, D.; Varani, G. Specificity of ribonucleoprotein interaction determined by RNA folding during complex formulation. Nature 1996, 380, 646–650.
- Levengood, J.; Tolbert, B. Idiosyncrasies of hnRNP A1-RNA recognition: Can binding mode influence function. Semin. Cell Dev. Biol. 2019, 86, 150–161.
- Dominguez, C.; Fisette, J.; Chabot, B.; Allain, F. Structural basis of G-tract recognition and encaging by hnRNP F quasi-RRMs. Nat. Struct. Mol. Biol. 2010, 17, 853–861.
- Dominguez, C.; Allain, F. Resonance assignments of the two N-terminal RNA recognition motifs (RRM) of the human heterogeneous nuclear ribonucleoprotein F (HnRNP F). J. Biomol. NMR 2005, 33, 282.
- Roca-Martínez, J.; Dhondge, H.; Sattler, M.; Vranken, W. Deciphering the RRM-RNA recognition code: A computational analysis. PLoS Comput. Biol. 2023, 19, e1010859.
- Chowdhury, M.; Jin, H. The RGG motif proteins: Interactions, functions, and regulations. Wiley Interdiscip. Rev. RNA 2023, 14, e1748.
- Thandapani, P.; O’Connor, T.; Bailey, T.; Richard, S. Defining the RGG/RG motif. Mol. Cell 2013, 50, 613–623.
- Lewis, H.; Musunuru, K.; Jensen, K.; Edo, C.; Chen, H.; Darnell, R.; Burley, S. Sequence-specific RNA binding by a Nova KH domain: Implications for paraneoplastic disease and the fragile X syndrome. Cell 2000, 100, 323–332.
- Yu, Q.; Ye, W.; Jiang, C.; Luo, R.; Chen, H. Specific recognition mechanism between RNA and the KH3 domain of Nova-2 protein. J. Phys. Chem. B 2014, 118, 12426–12434.
- Jensen, K.; Musunuru, K.; Lewis, H.; Burley, S.; Darnell, R. The tetranucleotide UCAY directs the specific recognition of RNA by the Nova K-homology 3 domain. Proc. Natl. Acad. Sci. USA 2000, 97, 5740–5745.
- Ghosh, M.; Singh, M. RGG-box in hnRNPA1 specifically recognizes the telomere G-quadruplex DNA and enhances the G-quadruplex unfolding ability of UP1 domain. Nucleic Acids Res. 2018, 46, 10246–10261.
- Braddock, D.T.; Baber, J.L.; Levens, D.; Clore, G.M. Molecular basis of sequence-specific single-stranded DNA recognition by KH domains: Solution structure of a complex between hnRNP K KH3 and single-stranded DNA. EMBO J. 2002, 21, 3476–3485.
- Görlich, D. Nuclear protein import. Curr. Opin. Cell Biol. 1997, 9, 412–419.
- Weighardt, F.; Biamonti, G.; Riva, S. The roles of heterogeneous nuclear ribonucleoproteins (hnRNP) in RNA metabolism. BioEssays News Rev. Mol. Cell. Dev. Biol. 1996, 18, 747–756.
- Rebane, A.; Aab, A.; Steitz, J. Transportins 1 and 2 are redundant nuclear import factors for hnRNP A1 and HuR. RNA 2004, 10, 590–599.
- Siomi, H.; Dreyfuss, G. A nuclear localization domain in the hnRNP A1 protein. J. Cell Biol. 1995, 129, 551–560.
- Edson, A.; Jacobsen, R.; Lewis, A. SAF-A/hnRNP U binds polyphosphoinositides via a lysine rich polybasic motif located in the SAP domain. microPubl. Biol. 2023, 2023.
- Miyaji, M.; Kawano, S.; Furuta, R.; Murakami, E.; Ikeda, S.; Tsutsui, K.; Tsutsui, K. Selective DNA-binding of SP120 (rat ortholog of human hnRNP U) is mediated by arginine-glycine rich domain and modulated by RNA. PLoS ONE 2023, 18, e0289599.
- Nozawa, R.; Boteva, L.; Soares, D.; Naughton, C.; Dun, A.; Buckle, A.; Ramsahoye, B.; Bruton, P.; Saleeb, R.; Arnedo, M.; et al. SAF-A Regulates Interphase Chromosome Structure through Oligomerization with Chromatin-Associated RNAs. Cell 2017, 169, 1214–1227.e18.
- Marenda, M.; Lazarova, E.; Gilbert, N. The role of SAF-A/hnRNP U in regulating chromatin structure. Curr. Opin. Genet. Dev. 2022, 72, 38–44.
- Caputi, M.; Zahler, A. Determination of the RNA binding specificity of the heterogeneous nuclear ribonucleoprotein (hnRNP) H/H’/F/2H9 family. J. Biol. Chem. 2001, 276, 43850–43859.
- Burd, C.; Dreyfuss, G. RNA binding specificity of hnRNP A1: Significance of hnRNP A1 high-affinity binding sites in pre-mRNA splicing. EMBO J. 1994, 13, 1197–1204.
- Görlach, M.; Burd, C.G.; Dreyfuss, G. The determinants of RNA-binding specificity of the heterogeneous nuclear ribonucleoprotein C proteins. J. Biol. Chem. 1994, 269, 23074–23078.
- Salehi, S.; Zare, A.; Prezza, G.; Bader, J.; Schneider, C.; Fischer, U.; Meissner, F.; Mann, M.; Briese, M.; Sendtner, M. Cytosolic Ptbp2 modulates axon growth in motoneurons through axonal localization and translation of Hnrnpr. Nat. Commun. 2023, 14, 4158.
- Chen, X.; Yang, H.; Zhang, B.; Phillips, J.; Cheng, D.; Rigo, F.; Witte, O.; Xing, Y.; Black, D. The RNA-binding proteins hnRNP H and F regulate splicing of a MYC-dependent HRAS exon in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2220190120.
- Alexander, M.; Hightower, R.; Reid, A.; Bennett, A.; Iyer, L.; Slonim, D.; Saha, M.; Kawahara, G.; Kunkel, L.; Kopin, A.; et al. hnRNP L is essential for myogenic differentiation and modulates myotonic dystrophy pathologies. Muscle Nerve 2021, 63, 928–940.
- Ran, Y.; Deng, Y.; Yao, C. U1 snRNP telescripting: Molecular mechanisms and beyond. RNA Biol. 2021, 18, 1512–1523.
- Zhang, S.; Aibara, S.; Vos, S.; Agafonov, D.; Lührmann, R.; Cramer, P. Structure of a transcribing RNA polymerase II-U1 snRNP complex. Science 2021, 371, 305–309.
- Choi, N.; Liu, Y.; Oh, J.; Ha, J.; Zheng, X.; Shen, H. U2AF65-Dependent SF3B1 Function in SMN Alternative Splicing. Cells 2020, 9, 2647.
- Von Voithenberg, L.V.; Sánchez-Rico, C.; Kang, H.; Madl, T.; Zanier, K.; Barth, A.; Warner, L.; Sattler, M.; Lamb, D. Recognition of the 3′ splice site RNA by the U2AF heterodimer involves a dynamic population shift. Proc. Natl. Acad. Sci. USA 2016, 113, E7169–E7175.
- Sharma, S.; Maris, C.; Allain, F.; Black, D. U1 snRNA directly interacts with polypyrimidine tract-binding protein during splicing repression. Mol. Cell 2011, 41, 579–588.
- Lan, C.; Zhang, H.; Wang, K.; Liu, X.; Zhao, Y.; Guo, Z.; Zhang, N.; Zhou, Y.; Gao, M.; Gu, F.; et al. The alternative splicing of intersectin 1 regulated by PTBP1 promotes human glioma progression. Cell Death Dis. 2022, 13, 835.
- Saulière, J.; Sureau, A.; Expert-Bezançon, A.; Marie, J. The polypyrimidine tract binding protein (PTB) represses splicing of exon 6B from the beta-tropomyosin pre-mRNA by directly interfering with the binding of the U2AF65 subunit. Mol. Cell. Biol. 2006, 26, 8755–8769.
- Izquierdo, J.; Majós, N.; Bonnal, S.; Martínez, C.; Castelo, R.; Guigó, R.; Bilbao, D.; Valcárcel, J. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell 2005, 19, 475–484.
- Matlin, A.J.; Southby, J.; Gooding, C.; Smith, C.W. Repression of α-actinin SM exon splicing by assisted binding of PTB to the polypyrimidine tract. RNA 2007, 13, 1214–1223.
- Liu, Y.; Kim, D.; Choi, N.; Oh, J.; Ha, J.; Zhou, J.; Zheng, X.; Shen, H. hnRNP A1 Regulates Alternative Splicing of Tau Exon 10 by Targeting 3′ Splice Sites. Cells 2020, 9, 936.
- Heiner, M.; Hui, J.; Schreiner, S.; Hung, L.; Bindereif, A. HnRNP L-mediated regulation of mammalian alternative splicing by interference with splice site recognition. RNA Biol. 2010, 7, 56–64.
- Turunen, J.; Verma, B.; Nyman, T.; Frilander, M. HnRNPH1/H2, U1 snRNP, and U11 snRNP cooperate to regulate the stability of the U11-48K pre-mRNA. RNA 2013, 19, 380–389.
- Oberstrass, F.; Auweter, S.; Erat, M.; Hargous, Y.; Henning, A.; Wenter, P.; Reymond, L.; Amir-Ahmady, B.; Pitsch, S.; Black, D.; et al. Structure of PTB bound to RNA: Specific binding and implications for splicing regulation. Science 2005, 309, 2054–2057.
- Wen, C.; Tian, Z.; Li, L.; Chen, T.; Chen, H.; Dai, J.; Liang, Z.; Ma, S.; Liu, X. SRSF3 and HNRNPH1 Regulate Radiation-Induced Alternative Splicing of Protein Arginine Methyltransferase 5 in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 14832.
- Eperon, I.; Makarova, O.; Mayeda, A.; Munroe, S.; Cáceres, J.; Hayward, D.; Krainer, A. Selection of alternative 5′ splice sites: Role of U1 snRNP and models for the antagonistic effects of SF2/ASF and hnRNP A1. Mol. Cell. Biol. 2000, 20, 8303–8318.
- Schaub, M.; Lopez, S.; Caputi, M. Members of the heterogeneous nuclear ribonucleoprotein H family activate splicing of an HIV-1 splicing substrate by promoting formation of ATP-dependent spliceosomal complexes. J. Biol. Chem. 2007, 282, 13617–13626.
- Thompson, M.; Muñoz-Moreno, R.; Bhat, P.; Roytenberg, R.; Lindberg, J.; Gazzara, M.; Mallory, M.; Zhang, K.; García-Sastre, A.; Fontoura, B.; et al. Co-regulatory activity of hnRNP K and NS1-BP in influenza and human mRNA splicing. Nat. Commun. 2018, 9, 2407.
- Ye, R.; Hu, N.; Cao, C.; Su, R.; Xu, S.; Yang, C.; Zhou, X.; Xue, Y. Capture RIC-seq reveals positional rules of PTBP1-associated RNA loops in splicing regulation. Mol. Cell 2023, 83, 1311–1327.e7.
- Shenasa, H.; Movassat, M.; Forouzmand, E.; Hertel, K. Allosteric regulation of U1 snRNP by splicing regulatory proteins controls spliceosomal assembly. RNA 2020, 26, 1389–1399.
- Erkelenz, S.; Mueller, W.; Evans, M.; Busch, A.; Schöneweis, K.; Hertel, K.; Schaal, H. Position-dependent splicing activation and repression by SR and hnRNP proteins rely on common mechanisms. RNA 2013, 19, 96–102.
- Khan, M.; Zhang, J.; Liu, Q. HnRNP F and hnRNP H1 regulate mRNA stability of amyloid precursor protein. Neuroreport 2021, 32, 824–832.
- Reznik, B.; Clement, S.; Lykke-Andersen, J. hnRNP F complexes with tristetraprolin and stimulates ARE-mRNA decay. PLoS ONE 2014, 9, e100992.
- Alarcón, C.R.; Goodarzi, H.; Lee, H.; Liu, X.; Tavazoie, S.; Tavazoie, S.F. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell 2015, 162, 1299–1308.
- Geissler, R.; Grimson, A. A position-specific 3′UTR sequence that accelerates mRNA decay. RNA Biol. 2016, 13, 1075–1077.
- McGlincy, N.; Tan, L.; Paul, N.; Zavolan, M.; Lilley, K.; Smith, C. Expression proteomics of UPF1 knockdown in HeLa cells reveals autoregulation of hnRNP A2/B1 mediated by alternative splicing resulting in nonsense-mediated mRNA decay. BMC Genom. 2010, 11, 565.
- Martinez, F.; Pratt, G.; Van Nostrand, E.; Batra, R.; Huelga, S.; Kapeli, K.; Freese, P.; Chun, S.; Ling, K.; Gelboin-Burkhart, C.; et al. Protein-RNA Networks Regulated by Normal and ALS-Associated Mutant HNRNPA2B1 in the Nervous System. Neuron 2016, 92, 780–795.
- Gratacós, F.; Brewer, G. The role of AUF1 in regulated mRNA decay. Wiley Interdiscip. Rev. RNA 2010, 1, 457–473.
- Lu, J.; Bergman, N.; Sadri, N.; Schneider, R. Assembly of AUF1 with eIF4G-poly(A) binding protein complex suggests a translation function in AU-rich mRNA decay. RNA 2006, 12, 883–893.
- Lu, J.; Schneider, R. Tissue distribution of AU-rich mRNA-binding proteins involved in regulation of mRNA decay. J. Biol. Chem. 2004, 279, 12974–12979.
- Yang, X.; Wen, Y.; Liu, S.; Duan, L.; Liu, T.; Tong, Z.; Wang, Z.; Gu, Y.; Xi, Y.; Wang, X.; et al. LCDR regulates the integrity of lysosomal membrane by hnRNP K-stabilized transcript and promotes cell survival. Proc. Natl. Acad. Sci. USA 2022, 119, e2110428119.
- Kim, D.; Kwak, E.; Kim, S.; Lee, K.; Woo, K.; Kim, K. hnRNP Q mediates a phase-dependent translation-coupled mRNA decay of mouse Period3. Nucleic Acids Res. 2011, 39, 8901–8914.
- Takahashi, T.; Ando, Y.; Ichikawa, H.; Tsuneyama, K.; Hijikata, T. Serum/glucose starvation strikingly reduces heterogeneous nuclear ribonucleoprotein A1 protein and its target, cyclin D1. FEBS J. 2023, 290, 4126–4144.
- Rajagopalan, L.; Westmark, C.; Jarzembowski, J.; Malter, J. hnRNP C increases amyloid precursor protein (APP) production by stabilizing APP mRNA. Nucleic Acids Res. 1998, 26, 3418–3423.
- Yugami, M.; Kabe, Y.; Yamaguchi, Y.; Wada, T.; Handa, H. hnRNP-U enhances the expression of specific genes by stabilizing mRNA. FEBS Lett. 2007, 581, 1–7.
- Lee, D.; Lim, M.; Youn, D.; Jung, S.; Ahn, Y.; Tsujimoto, Y.; Lee, J. hnRNP L binds to CA repeats in the 3′UTR of bcl-2 mRNA. Biochem. Biophys. Res. Commun. 2009, 382, 583–587.
- Percipalle, P.; Raju, C.; Fukuda, N. Actin-associated hnRNP proteins as transacting factors in the control of mRNA transport and localization. RNA Biol. 2009, 6, 171–174.
- Andreou, M.; Yan, C.; Skourides, P. 40LoVe and Samba are involved in Xenopus neural development and functionally distinct from hnRNP AB. PLoS ONE 2014, 9, e85026.
- Raju, C.; Fukuda, N.; López-Iglesias, C.; Göritz, C.; Visa, N.; Percipalle, P. In neurons, activity-dependent association of dendritically transported mRNA transcripts with the transacting factor CBF-A is mediated by A2RE/RTS elements. Mol. Biol. Cell 2011, 22, 1864–1877.
- Kroll, T.; Swenson, L.; Hartland, E.; Snedden, D.; Goodson, H.; Huber, P. Interactions of 40LoVe within the ribonucleoprotein complex that forms on the localization element of Xenopus Vg1 mRNA. Mech. Dev. 2009, 126, 523–538.
- Raju, C.; Göritz, C.; Nord, Y.; Hermanson, O.; López-Iglesias, C.; Visa, N.; Castelo-Branco, G.; Percipalle, P. In cultured oligodendrocytes the A/B-type hnRNP CBF-A accompanies MBP mRNA bound to mRNA trafficking sequences. Mol. Biol. Cell 2008, 19, 3008–3019.
- Fukuda, N.; Fukuda, T.; Sinnamon, J.; Hernandez-Hernandez, A.; Izadi, M.; Raju, C.S.; Czaplinski, K.; Percipalle, P. The Transacting Factor CBF-A/Hnrnpab Binds to the A2RE/RTS Element of Protamine 2 mRNA and Contributes to Its Translational Regulation during Mouse Spermatogenesis. PLoS Genet. 2013, 9, e1003858.
- Van Dusen, C.; Yee, L.; McNally, L.; McNally, M. A glycine-rich domain of hnRNP H/F promotes nucleocytoplasmic shuttling and nuclear import through an interaction with transportin 1. Mol. Cell. Biol. 2010, 30, 2552–2562.
- Izaurralde, E.; Jarmolowski, A.; Beisel, C.; Mattaj, I.; Dreyfuss, G.; Fischer, U. A role for the M9 transport signal of hnRNP A1 in mRNA nuclear export. J. Cell Biol. 1997, 137, 27–35.
More
