Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Umesh K. Reddy and Version 2 by Lindsay Dong.
Drosophila melanogaster, an invertebrate model with its extensively studied genome, has more than 70% gene homology to humans and has been used as a model system in biological studies for a long time. The notable advantages of Drosophila as a model system, such as their low maintenance cost, high reproductive rate, short generation time and lifespan, and the high similarity of metabolic pathways between Drosophila and mammals, have encouraged the use of Drosophila in the context of screening and evaluating the impact of phytochemicals present in the diet.
- Drosophila
- phytochemical
- human health
- metabolism
- disease
1. Introduction
Phytochemicals are specialized metabolites with biological properties stored in plant tissues, such as roots, stems, leaves, flowers, and fruits [1]. Phytochemicals include a wide range of compounds such as polyphenols, carotenoids, flavonoids, coumarins, terpenoids, glucosinolates, saponins, and capsaicinoids, which are often associated with the vibrant colors of fruits and vegetables [2]. Although phytochemicals are not essential nutrients in plants, they are responsible for many health benefits associated with a plant-based diet [3][4][3,4]. Potential phytochemical health benefits include antioxidant, anti-inflammatory, anti-cancer, and anti-microbial properties [5]. Currently, numerous phytochemicals are being studied for their possible use in developing novel drugs and dietary supplements [6].
Phytochemicals exhibit their beneficial and harmful effects by interaction with multiple cell signaling molecules [7]. Nevertheless, the molecular mechanisms underlying the effectiveness of phytochemicals continue to grow. In this context, employing cost-effective, rapid, reliable, and efficient in vitro and in vivo assays will facilitate analyzing these compounds’ metabolic processes, dosage response, and pharmacological and toxicological profiles [8][9][10][8,9,10]. D. melanogaster, known as the fruit fly, has emerged as an alternate animal model. Due to its short lifespan, small size, and well-understood genome, it has been widely used to examine the efficacy and safety of phytochemicals on various physiological processes, including metabolism, aging, and immunity [11][12][11,12].
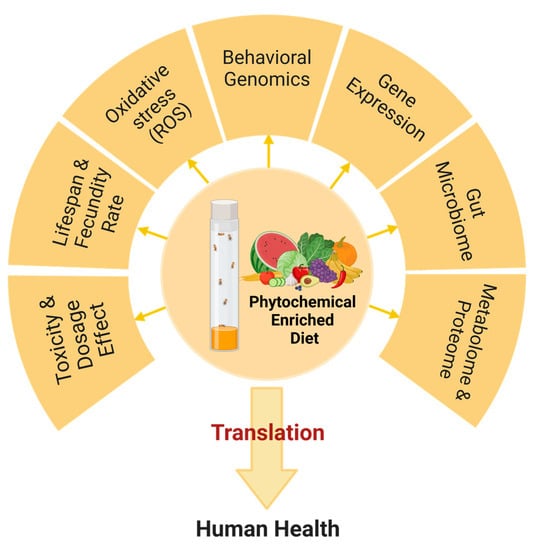
2. Phytochemicals and Their Potential Therapeutic Benefits
Regular consumption of fruits, vegetables, and grains has been associated with a reduced risk of certain chronic diseases due to phytochemicals with antioxidant and anti-inflammatory properties [13][19]. Phytochemicals regulate oxidative stress, which has been recognized as a significant factor in the pathogenesis of metabolic disorders and cancer [14][20]. Phytochemical therapeutic benefits are divided mainly into five categories (i) enhancers of the body’s immune system; (ii) preventers of diabetes and heart diseases; (iii) hypocholesterolemic agents; (iv) promoters of digestion and absorption; and (v) retardants of aging [15][16][21,22]. The major classes of phytochemicals with disease-preventing functions are dietary fiber, antioxidants, detoxifying agents, immunity-potentiating, and neuropharmacological agents [17][23]. For instance, polyphenols such as resveratrol have been associated with decreased risk of myocardial infarction, stroke, and diabetes [18][19][24,25]. Polyphenols in diet also help to improve lipid profiles, blood pressure, insulin resistance, and systemic inflammation [20][26]. Furthermore, vitamin C and carotenoids may benefit immune function, thereby reducing cancer risk by enhancing the tumor surveillance of the immune system [21][27]. Capsaicinoids, including capsaicin and dihydrocapsaicin found in peppers, have been found to have beneficial roles in humans, including managing pain inflammation during rheumatoid arthritis, anti-cancer agent by generating reactive oxygen species, and in the prevention or treatment of neurodegenerative diseases such as Alzheimer’s disease due to its antioxidant activity [22][23][24][28,29,30]. Other spices, such as turmeric obtained from the roots of Curcuma domestica, contain a yellow coloring principle, curcumin, a powerful antioxidant that can offer protection against cancer, inhibiting lipid peroxide-induced DNA damage [25][35]. Flavonoid consumption, such as quercetin and kaemferol, through vegetables and fruits, reduces the risk of death from coronary heart disease [26][36].3. Advantages of Using Drosophila as a Translational Model for Testing Phytochemicals
For decades, D. melanogaster has been widely used as an excellent animal model to study genetics, evolution, and developmental biology [27][39]. It is a cost-effective option due to its high reproductive rate (30–50 eggs/day), short generation time (approximately ten days at 25 °C), and low maintenance cost. In addition, Drosophila short lifespan (average three months at 25 °C) and easy generation of large populations allow for performing longevity and lifespan assays in only a few months [28][40]. Furthermore, it offers powerful molecular and genetic tools that permit gene overexpression or knock-down studies [29][41]. Although Drosophila is evolutionarily distant from humans, fly development, physiological, biological, and metabolic processes are equivalent to many of those found in mammals. Recently, similarities between humans and fruit flies in terms of metabolic regulation, including the role of insulin signaling, nutrient sensing, and energy homeostasis in metabolic disorders, such as diabetes and obesity [30][31][32][33][42,43,44,45]. Moreover, ingesting complex foods rich in phytochemicals by an organism leads to the degradation of nutrients that directly affect the gastrointestinal microbiome because the host and microbiome share the same food source [34][46]. These microbiome changes influence the organism’s phenotype and behavior by altering the genome, transcriptome, epigenome, proteome, and metabolome [35][47].4. Methods for Testing the Efficacy of Phytochemicals in Drosophila
Phytochemical ingestion effects are observed in general physiology, including metabolism, behavior, stress resistance, reproductive capacity, nervous system, and immune capacity in both Drosophila and humans, and aspects of these physiological changes can be used as parameters to determine the phytochemical effects and toxicity [36][50]. Among them, lifespan and survival rate are simple and efficient longitudinal assays to determine the effects after administration of the candidate plant-based compounds [37][53]. In addition, various strains with different lifespan characteristics and transgenic flies with symptoms similar to human diseases are available to evaluate plant compounds’ effects on mortality rate. Oxidative stress mitigation in Drosophila is also an essential parameter in assessing the efficacy of phytochemicals. Levels of reactive oxygen species (ROS), activities of antioxidant enzymes, such as superoxide dismutase and catalase, and lipid peroxidation in treated fly cells provide insight into the potential antioxidative activities of phytochemicals (Figure 1) [38][56]. Moreover, Drosophila has a relatively simple nervous system, making it an ideal model to study neurodevelopment and neurodegeneration using automated tracking systems, locomotor activity, ring assay, gustatory, social, and circadian rhythm patterns, providing insight into potential impacts of phytochemicals on the nervous system function and behavior [39][57].
Figure 1.
Approaches for evaluating the effects of phytochemicals in
Drosophila melanogaster
model.
Although administration of compounds via feeding is the most typical method of phytochemical delivery to Drosophila [36][50], it is important to consider several concerns about feeding-related artifacts, such as reduced feeding related to the preference of flies for each compound, uncertainty about the amount of food consumed by each individual and of the actual phytochemical concentration achieved in the tissues of the flies.
5. Studies Evaluating the Effect of Phytochemicals by Using Drosophila
5.1. Phytochemical Effect on Aging
Anti-aging research has gained significant attention due to the growing aging population, which experiences a natural decline in the body’s capacity to repair molecular, biochemical, and organ damage, resulting in increased susceptibility to age-related diseases [40][60]. The primary cause of radical damage to macromolecules in aging is the progressive decline of the endogenous antioxidant system, triggered by reactive oxygen species (ROS). To restore the ROS balance, natural plant-derived antioxidants can be utilized to reinforce the endogenous antioxidant system [41][61].
Plant-derived compounds with anti-aging properties have been investigated. Moringa oleifera has been identified as one of the plants with the highest levels of bioactive molecules, such as polyphenols, flavonoids, and tannins, which elicit an antioxidant response [42][62].
Withanolides are widely found in Solanaceae species and have been investigated for their potential to enhance resilience against age-related stress [43][65]. Withania somnifera extracts with high withanolides concentration have been shown to ameliorate behavioral deficits in an in vivo D. melanogaster model of oxidative stress, reducing the effects of aging in locomotion and cognition [44][66].
5.2. Development and Lifespan
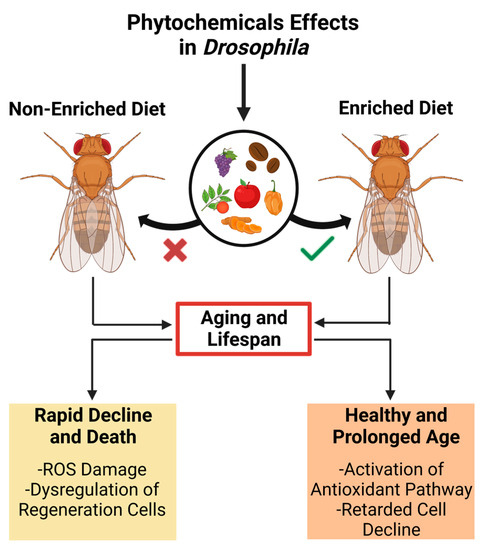
Age-related dysregulation of development is closely associated with functional organ and tissue decline, affecting lifespan and age-related disease development [40][60]. A modern lifestyle characterized by high caloric intake and minimal physical activity in humans results in high lipid storage levels that reduce overall lifespan [45][68]. Nevertheless, several studies have shown that several phytochemicals can expand the lifespan using Drosophila as a model (Figure 2) [46][69].
Figure 2. The impact of a phytochemical-enriched diet on the lifespan of Drosophila melanogaster suggests potential applications for promoting healthy aging and longevity in humans.
5.3. Metabolism
Recent evidence suggests that the metabolic state of an organism is closely tied to its diet, with an important factor being that the dietary habits of parents can impact the metabolic states of their offspring [47][77]. Controlled dietary conditions are crucial for studying metabolism and organism physiology [48][78]. Drosophila possesses notable metabolic systems that share many conserved functions with vertebrates, including insulin, insulin-like growth factor, the target of rapamycin signaling pathways, and energy regulation [49][50][79,80].
Obesity is a prevalent metabolic syndrome in humans, leading to various metabolic complications, such as impaired glucose tolerance, insulin resistance, dyslipidemia, hypertension, type 2 diabetes, and premature heart disease [51][81]. Heinrichsen et al. [52][82] evaluated the metabolic response of Drosophila fed a high-fat diet, which resulted in increased levels of triglyceride and glucose, decreased stress tolerance and lifespan, and activation of pathways associated with fat metabolism, insulin signaling, cardiac fat accumulation, and dysfunction.
Moreover, the effects of resveratrol were also analyzed in Drosophila metabolism. It was observed that resveratrol supplementation improved metabolic parameters, such as enhanced glucose tolerance and reduced lipid accumulation. Nevertheless, the implementation of target-specific therapy might be beneficial in mitigating any negative consequences arising from the pro-oxidant activity associated with high dosages [53][87]. Meanwhile, quercetin supplementation, a flavonoid in various fruits and vegetables, improved glucose homeostasis, reduced oxidative stress, and enhanced mitochondrial function in flies [54][88].
5.4. Microbiome
The gut microbiome in the human intestines plays a critical role in nutrient absorption, lysis, and storage [55][94]. Additionally, they are essential for various physiological processes, including metabolism, digestion, circadian rhythms, and vitamin synthesis in humans [56][95]. Imbalances in the gut microbiota (dysbiosis) due to diet, antibiotic use, age, and stress contribute to disease development, including diabetes, obesity, colon cancer, inflammatory bowel disease, inflammation, and neurodegeneration [57][96]. The Drosophila gut microbiome is extracellular and encompasses three regions: the foregut, midgut, and hindgut, each creating distinct conditions for microbial cells [58][97]. Although the Drosophila gut microbiome has been well-documented, it is important to note that fruit flies have a limited number of microorganisms, about 30 species, compared to mammals with more than 500 species [59][98]. The gut microbial population is profoundly influenced by the dietary habits associated with consuming different types and amounts of phytochemicals (99). It also varies depending on the host genotype, age, sex, and habitat [60][61][99,100]. Moreover, Jimenez-Padilla et al. [62][103] found that Drosophila flies fed diets with strawberries and blueberries had an increased abundance of Acetobacter in their microbiome. Acetobacter, particularly Acetobacter pomorum, has probiotic properties and produces acetic acid, which promotes insulin signaling, reducing lipid and sugar levels in adult flies [63][104]. The blueberry diet also led to higher levels of Actinobacteria compared to the control diet [62][103]. Actinobacteria, including the genus Bifidobacterium, metabolize anthocyanins into small compounds with probiotic effects for obesity and gastrointestinal and systemic diseases [64][65][105,106].5.5. Neurodegenerative Diseases
D. melanogaster has been widely used in drug screening studies to identify high-quality hits that exhibit crucial characteristics, including metabolic stability, oral or transdermal availability, and, most notably, low toxicity, providing a valuable resource for drug development [28][40]. Phytochemicals have been extensively studied for their potential in preventing and controlling the proliferation and development of tumor cells [66][108]. Black beans, specifically Phaseolus vulgaris, contain phenolic compounds, including cyanidin-3-O-glucoside (C3G), recognized as an anti-cancer compound [67][109]. Likewise, phytochemicals can offer neuroprotection, which is crucial for developing new treatments for neurodegenerative diseases, such as Alzheimer’s. In Alzheimer’s disease, memory loss is associated with the forming beta-amyloid plaques, which give rise to oligomers that generate reactive oxygen species (ROS) and promote Tau protein aggregation, ultimately leading to neuronal cell death [68][111]. Moreover, epilepsy is a neurological disorder characterized by sensory-motor deficits and convulsions. It can have various causes, including gene mutations that encode ion channels in brain cells responsible for transmitting signals between neurons [69][117]. To alleviate epilepsy symptoms, researchers have investigated using Imperata cylindrica root extracts in a mutant Drosophila “para” gene model. The treated flies exhibited an inhibitory effect on voltage-gated sodium ion channels, which reduced inflammation and increased tissue repair in brain cells, confirming the extract’s efficacy in treating epilepsy [69][117].6. Gene Regulation Induced by Phytochemicals in Drosophila
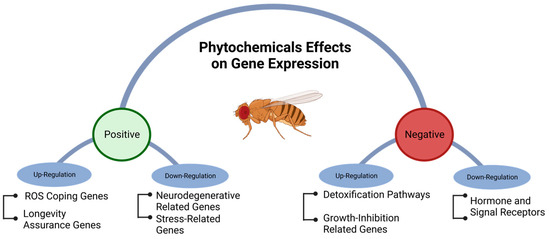
Phytochemical intake can modulate gene expression, influenced by factors, such as cell type, life stage, and growing conditions. Different mechanisms of gene regulation, including cis-regulatory elements, repressor proteins, non-coding RNA, and epigenetic processes, such as methylation, may be affected by phytochemicals [70][71][72][73][93,118,119,120]. High-throughput technologies, such as RNA-seq, are commonly used to study gene regulation and metabolic pathways on a genome-wide scale [73][74][120,121]. Phytochemicals have the potential to modulate metabolic pathways and coping mechanisms that counteract age-related neurodegenerative diseases [75][122]. Although phytochemicals can positively influence the health of D. melanogaster by upregulating genes associated with longer lifespans and down-regulating genes related to diseases and stress, they can also have negative effects by upregulating genes associated with reduced growth rates. Additionally, they may trigger mechanisms related to detoxification and reduce the expression of genes involved in coping with reactive oxygen species (ROS) and hormone-signaling receptors (Figure 34).
Figure 4.
Positive and negative genetic regulation of metabolic pathways in
Drosophila melanogaster
exposed to phytochemicals.
7. Conclusions
Plant extracts have long been employed for their therapeutic and preventive properties in addressing various disorders. These extracts encompass a wide array of bioactive compounds, including polyphenols, carotenoids, flavonoids, curcuminoids, terpenoids, and capsaicinoids, contributing to their potential beneficial effects. Drosophila is an excellent model organism with extensive use in studying diverse biological processes. Leveraging the vast range of powerful genetic and molecular biology tools available, the Drosophila model offers a valuable and cheap alternative for investigating the effects of plant extracts and their derived compounds on large populations in a short period.
