2. Obesity Treating Natural Products
2.1. Single Compound
One study with a single compound showed antiobesity effects (
Table 1). Diethyl azelate (DEA) is naturally produced in animals and plants and can be used to improve related metabolic syndromes
[8]. Steeper et al. reported that daily oral DEA decreased total cholesterol (TC) and low-density lipoprotein (LDL) levels in human males who were overweight, alleviating obesity. This study on DEA included 17 participants and lasted for 21 days. More reliable results would have been drawn if this study had enrolled more subjects. This study’s design also decreased its reliability; it used a 21-day prospective design in a before–after clinical trial and did not use blinding or a placebo control during treatment.
Table 1. Single compound.
TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein. ↓, decrease.
It was impossible to determine the trend of studies regarding the antiobesity effects of single compounds because there was only one study in this category.
2.2. Foods
Twenty-six human studies examined using foods to treat obesity (
Table 2).
LDL, low-density lipoprotein; BW, body weight; HDL, high-density lipoprotein; TC, total cholesterol; TG, triacylglycerols (triglyceride); ApoB, apolipoprotein B; ApoA, apolipoprotein A; NPY, neuropeptide Y; BF, body fat; HMW, high molecular weight; APN, adiponectin; VLDL, very-low-density lipoprotein; PUFA, polyunsaturated fatty acid; L:A, leptin:adiponectin; VFA, visceral fat area; FPG, fasting plasma glucose; ↓, decrease; ↑, increase.
C. carvi L. (caraway) aqueous extract (CAE) decreased WC, waist-to-hip ratio (WHR), thigh circumference (THC), and mid-upper arm circumference
[12]. Rondanelli et al. demonstrated that
C. scolymus (artichoke) decreased visceral adipose tissue (VAT), fat mass (FM), and WC
[14]. These results demonstrated that artichokes could potentially treat individuals with overweight and impaired fasting glucose.
V. vinifera L. (grape) seed extract (GSE) decreased several anthropometric measurements, including BW, BMI, WC, hip circumference (HC), and WHR, demonstrating its potential to treat obesity
[16]. The treatment group received GSE (300 mg/day) for 12 weeks, also lowering neuropeptide Y (NPY) levels compared to the placebo group. An
L. plantarum fermented barley–wheat flour compound noodle (FBWN) decreased WC, fat rate, FM, and visceral fat (VF) and increased muscle mass and basal metabolic rate
[17]. Boix-Castejón et al. reported that combining
L. citriodora (lemon beebrush) and
H. sabdariffa (roselle; LC-HS) decreased appetite and attraction to fatty, sweet, and salty foods, decreasing obesity
[18].
Matured
H. lupulus L. (hop) bitter acids attenuated diet-induced body fat (BF) accumulation in rodents by enhancing thermogenesis in brown adipose tissue (BAT) through the activity of sympathetic nerves innervating BAT
[19]. Morimoto-Kobayashi et al. reported that matured hop extract reduced total fat area primarily by reducing VF area (VFA). Oniki et al. reported that
G. gnemon Linn. (melinjo) seed extract (MSE) activated genes regulating APN multimerization. It has been demonstrated that APN may enhance insulin sensitivity and protect against obesity, type 2 diabetes, and atherosclerosis
[20]. Rao et al. reported that
N. sativa (black seed or jintan hitam) and
Trigonella foenum-graecum (fenugreek) supplemented chapatis (NFCs) also decreased BM, BMI, WC, HC, and the central obesity index
[21]. Kim et al. reported that
P. grandiflorus (balloon flower) ethanol extract (PGE) reduced BFM and BF percentage (BFP)
[23]. PGE571 (PGE at 571 mg) decreased leptin levels, BFM, and BFP and increased muscle mass. PGE2855 (PGE at 2855 mg) decreased the leptin:APN ratio, BFM, BFP, and total abdominal and subcutaneous fat areas.
Nishimura et al. reported that quarantine-rich onion did not decrease the VFA
[24]. Nevertheless, participants with low HDL-C levels in the quercetin-rich onion group showed significantly lower VFAs. Amini et al. reported that
S. officinalis (common sage) decreased BW, BMI, and WC
[25]. Common sage extract at 330 mg/day for eight weeks positively affected lipid metabolism. Maia-Landim et al. reported that standardized
G. cambogia (Malabar tamarind) extracts (52.4% hydroxycitric acid (HCA)) and
A. konjac (konjac; 94.9% glucomannan) decreased plasma glucose, cholesterol, and TG levels; FM; VF; and BW and increased the basal metabolic rate
[26]. However, polymorphisms in perilipin 4 (
PLIN4; −11482G > A), FM and obesity-associated (
FTO; rs9939609 (A/T)), and β-adrenergic receptor 3 (
ADRB3; Trp64Arg) attenuated its lipolysis effect. Farhat et al. reported that
S. rebaudiana (stevia) intake did not result in energy compensation during lunch or throughout the day and reduced postprandial glucose levels compared to sugar
[27]. Stevia was found to lower appetite and stop the increase in food intake. Leverrier et al. reported that 500 mg/day of
H. annuus (sunflower) seed extract for 12 weeks decreased cholesterol, long-lasting LDL, BW, BMI, and WC
[28]. The intervention was especially effective in females with obesity aged >30 years.
Six weeks of
C. lanatus (watermelon) supplementation increased fasting plasma L-arginine, cis-lycopene, and trans-lycopene levels and decreased vascular cell adhesion molecule 1 (VCAM1) levels
[29]. This study only suggested indirect effects on obesity, so further research is needed to obtain effective results for lipid metabolism. A new comprehensive study by Permatasari et al. showed that
C. racemosa (green seaweed or green algae) could be a new candidate for antiobesity functional food
[30]. This study integrated in silico and in vitro experiments with a four-week, randomized, double-blind, placebo-controlled clinical trial. A randomized, double-blind, parallel-group, placebo-controlled pilot study by Majeed et al. demonstrated the antiobesity potential of
C. rotundus extract (CRE)
[31]. Interestingly, CRE showed antiadipogenic activity, was safe for human consumption, and effectively managed weight and hypercholesterolemia in individuals with overweight.
The main active ingredient in Malabar tamarind extract is HCA, which is known to attenuate weight gain and fat synthesis in animals and humans
[32]. However, the mechanism underlying the action of HCA is not fully understood. A three-month clinical study on 100 individuals with obesity and a subsequent computational study investigated the effect of HCA treatment on anthropometric measurements and plasma lipid profiles in human subjects
[32]. They showed that HCA could reduce weight gain and fat accumulation in subjects with obesity. Han et al. conducted a randomized, double-blind, placebo-controlled study assessing the effect of standardized
H. serrata (Thunb.) Ser. leaf extract (WHS) on BW and BF reduction in human subjects with overweight or obesity
[33]. Daily WHS supplementation reduced BW, BMI, and BFM. Interestingly, this was accompanied by reduced HC, VFA, abdominal fat area, and the visceral–subcutaneous ratio. More interestingly, no significant side effects were observed during or after 12 weeks of this intervention.
All the above studies support the claim that certain foods help prevent obesity. Foods used in these studies were usually also treated as herbal medicines, and many processed foods into extracts to test their effects on obesity. Certain foods reduce obesity usually by controlling metabolic hormones or reducing appetite. Most studies stated that there were no side effects. However, some studies used noodle or snack forms to test the food’s antiobesity effect
[13][17][13,17]. Moreover, some studies did not clearly indicate a mechanism for reducing obesity. Therefore, further studies are needed.
2.3. Teas
Twelve human studies treated obesity using tea (
Table 3). Yonekura et al. conducted a cross-sectional study on
C. arabica (coffee) and
C. sinensis (green tea). These substances were administered to 232 Japanese women aged 40–65 years with menopausal symptoms who completed the brief-type self-administered diet history questionnaire
[35]. Patients were divided into four groups depending on their coffee (CF) and green tea (GT) consumption. Using a multivariate model, they showed an inverse relationship between daily CF/GT intake and BW, BMI, and cardio-ankle vascular index. Ghasemi et al. conducted a clinical trial using combined high-intensity interval training and green tea supplementation in 30 women with overweight
[36]. They determined that daily green tea consumption increased the levels of sirtuin 1 (SIRT1), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and catalase (CAT) and significantly decreased BFP, BMI, and BW. Therefore, the catechins in green tea inhibit lipogenesis, increase fat oxidation, and improve antioxidant capacity. Kobayashi et al. conducted a randomized, double-blind, placebo-controlled trial examining the effectiveness of green tea beverages enriched with catechins and a galloyl moiety on obesity in 124 subjects with obesity
[37]. Green tea catechins with a galloyl moiety reduced BW, BMI, and BFP by decreasing abdominal fat area via inhibiting or attenuating intestinal fat absorption.
BW, body weight; BMI, body mass index; VAT, visceral adipose tissue; LDL, low-density lipoprotein; FFA, free fatty acid; PGC-1α, proliferator-activated receptor gamma coactivator 1-alpha; BF, body fat; FO, fat oxidation; ↓, decrease; ↑, increase.
All these studies support the view that tea is effective in weight loss. Most studies supported green tea’s ability to help individuals lose weight; only one study found the beverage ineffective. Therefore, further research on the obesity-reducing effect of green tea is needed. In addition to green tea, coffee, kosen-cha, oolong tea, and puer tea were reported to alleviate obesity.
2.4. Fruits
Six studies demonstrated the effectiveness of fruit-derived natural products in ameliorating obesity (
Table 4). Duchnowicz et al. reported that
A. melanocarpa decreased acetylcholinesterase (AChE) activity and oxidative stress, improving lipid metabolism related to cholinesterase activity
[46].
A. melanocarpa at 3 × 100 mg/day for two months decreased cholesterol and lipid peroxidation, reducing AChE. Rondanelli et al. found that bergamot phytosome positively affected VAT after 30 days and remained effective for a further 60 days
[47]. Bergamot phytosome tablets (500 mg) taken twice daily for 12 weeks modulated lipids, decreasing TC and LDL and increasing HDL. All these studies support the efficacy of fruit-derived natural products against obesity and lipid disorders, although there were some limitations. Treatments in several studies appeared effective but were not significant. In addition, a few studies were conducted on obesity-related bioavailability, such as metabolic disorders, inflammatory status, and antioxidant capacity, rather than on obesity itself.
Table 4. Fruits.
| Extract |
Study Design |
Population |
Status |
Number |
Outcome |
Lab Test |
Reference |
| Aronia melanocarpa extract |
Placebo-controlled trial |
77 |
Completed |
|
Decreased cholinesterase activity |
↑ HDL, cholesterol, TAC “fast” parameter; ↓ TC, LDL, TG, TAC “slow” parameter, lipid peroxidation, cholesterol in the erythrocyte membranes |
[46] |
| Citrus bergamia (bergamot) phytosome |
Randomized, double-blind, placebo-controlled trial |
64 |
Completed |
|
Decreased VAT |
↓ TC, LDL, ApoB, LDL/HDL;
↑ ApoA/HDL |
[47] |
| |
[ | 39 | ] |
| Citrus bergamia (bergamot) polyphenol extract-complex |
Randomized, double-blind, placebo-controlled trial |
45 |
Completed |
UNICZ Trial No. 182/2016 |
Green coffee bean extract |
Randomized, double-blind, placebo-controlled clinical trial |
64 |
Completed |
|
Decreased obesity |
↑ Serum adiponectin; ↓ total serum cholesterol, LDL, FFA, leptin |
[40] |
| Randomized, double-blind, placebo-controlled trial |
98 |
Completed |
Green tea |
10-week randomized, placebo-controlled trial |
30 |
Completed |
NCT04950062 |
Increased metabolic status |
↑ PGC-1α |
[36] |
| |
Decreased cholesterol and BW |
↓ LDL |
Green tea |
Randomized, double-blind, placebo-controlled clinical trial |
124 |
Completed |
|
Decreased BF |
|
[37] |
| Decreased weight |
↓ TC, LDL, TAG, serum leptin, serum ghrelin; |
| ↑ HDL, serum adiponectin |
[ | 48 | ] |
[49] |
| Grape pomace and Schisandra chinensis (omija) fruit ethanol extract |
Randomized, double-blind, placebo-controlled trial |
76 |
Completed |
|
Decreased obesity-related dyslipidemia |
High GO: ↑ ApoA-1; ↓ TC, non-HDL-C, LDL-C, plasma ApoB, Apo B/ApoA-1 ratio, plasma Lp(a) |
[50] |
| Euterpe edulis (juçara) pulp powder |
Randomized, double-blind trial |
35 |
Completed |
RBR-5RXR2B |
Decreased obesity |
↑ HDL-C, serum adiponectin; ↓ TC, LDL, TAG, L:A ratio |
[51] |
Randomized double-blind placebo-controlled nutritional intervention clinical trial with two parallel arms |
92 |
| Garcinia mangostana | |
(mangosteen) extract | DRKS00010533 |
Decreased obesity |
Green tea extract | ↓ LDL-C |
Double-blinded placebo-controlled trial |
26-week prospective randomized, controlled, parallel-group study |
20 |
Completed[15] |
| 45 |
Completed |
IRCT20151025024699N3 |
Decreased obesity |
↑ Adiponectin, irisin |
[ | 41 | ] |
NCT02823561 |
Decreased weight |
↓ HDL |
[52] |
Vitis vinifera L. (grape) seed extract |
Randomized, double-blind, placebo-controlled clinical trial |
40 |
Completed |
IRCT2015073015968N3 |
Decreased obesity |
↓ NPY |
[16] |
| High-dose green tea extract (epigallocatechin gallate) |
Randomized, single-center, placebo-controlled, double-blind study |
77 |
Unknown |
NCT02147041 |
| Citrus bergamia (bergamot) |
Decreased weight |
↑ Adiponectin; |
| ↓ cholesterol, LDL, ghrelin |
[ | 42 | ] |
Lactobacillus plantarum fermented Hordeum vulgare-Triticum aestivum (barley-wheat) flour compound noodle |
Single-blinded, controlled, parallel clinical trial |
30 |
Completed |
ChiCTR1800019614 |
Decreased obesity |
| Kosen-cha | ↓ TG |
12-week, prospective, before–after study | [ |
6 |
Completed |
|
Decreased obesity |
↓TG, ↑insulin sensitivity17] |
| [ | 43 | ] |
Lippia citriodora (lemon beebrush) and Hibiscus sabdariffa (roselle) |
8-week, randomized, double-blind, placebo-controlled clinical trial |
54 |
Completed |
|
Decreased obesity, appetite |
↓ Leptin, resistin |
| Oolong tea | [ | 18 | ] |
| 14-day, placebo-controlled, double-blind, crossover intervention trial |
Matured Humulus lupulus L. (hops) |
Randomized, double-blind, placebo-controlled parallel-arm clinical trial |
178 |
Completed |
UMIN000014185 |
Decreased BF |
|
[19] |
| Gnetum gnemon Linn (melinjo) seed |
Prospective, randomized, parallel, double-blind, placebo-controlled clinical trial |
42 |
Completed |
UMIN000025643 |
Increased APN multimerization |
↑ HMW/total APN ratio |
[20] |
| Nigella sativa (black seed or jintan hitam) and Trigonella foenum-graecum (fenugreek) supplemented chapatis |
12-week prospective, before–after clinical trial |
40 |
Completed |
|
Decreased obesity |
↓ TC, non-↑ HDL-C, VLDL, TG, ↓ HbA1C, FPG |
[21] |
| Allium cepa L. (onion) peel |
Randomized, double-blind, placebo-controlled clinical trial |
61 |
|
|
Decreased obesity |
↑ PUFA n-6
↓ PUFA n-3 |
[22] |
| Platycodon grandiflorus (balloon flower) ethanol extract |
Single-center, randomized, double-blind, placebo-controlled clinical trial |
72 |
Completed |
|
Decreased obesity |
PGE571: ↓ leptin.
PGE2855: ↓ L:A ratio |
[23] |
| Quercetin-rich Allium cepa L. (onion) powder |
Randomized, double-blind, placebo-controlled, parallel-group clinical trial |
54 |
Completed |
UMIN000033410 |
Subjects with lower HDL-C: decreased VFA. |
|
[24] |
| Salvia officinalis (common sage) |
Randomized triple-blinded placebo-controlled clinical trial |
60 |
Completed |
IRCT201504146917N2 |
Decreased obesity |
|
[25] |
| Garcinia cambogia (Malabar tamarind) and Amorphophallus konjac (konjac) |
Prospective, nonrandomized controlled intervention clinical trial |
214 |
Completed |
|
Decreased weight |
↓ Cholesterol, TG |
[26] |
| Stevia rebaudiana (stevia) |
Randomized, three-arm, single-blinded crossover clinical trial |
30 |
Completed |
NCT01115088 |
Decreased energy intake |
|
[27] |
| Helianthus annuus (sunflower) seed extract |
Randomized, placebo-controlled, double-blind, parallel-group clinical pilot study |
46 |
Completed |
|
Decreased obesity |
↓ Cholesterol, long-lasting LDL |
[28] |
| Citrullus lanatus (watermelon) |
Randomized 2-arm design with a single 6-week intervention period |
45 |
Completed |
NCT04015544 |
Decreased obesity |
|
[29] |
| Caulerpa racemosa (green algae) |
Randomized, double-blind, placebo-controlled clinical trial |
74 |
Completed |
NCT05037591 |
Decreased obesity |
↑ HDL, proliferator-activated receptor-γ coactivator α (PGC-1α);
↓ TC, TG |
[30] |
HDL, high-density lipoprotein; TAC, total antioxidant capacity; TC, total cholesterol; LDL, low-density lipoprotein; TG, triacylglycerols; VAT, visceral adipose tissue; ApoB, apolipoprotein B; ApoA, apolipoprotein A; TAG, triacylglycerols; BW, body weight; ApoA-1, apolipoprotein A-1; Lp(a), lipoprotein(a); L:A ratio, leptin-to-adiponectin ratio; ↓, decrease; ↑, increase.
Obesity is a global burden transcending borders with continuously high prevalence rates [53][75]. While current technologies and synthetic medicines are being adopted to treat obesity, their related complications and safety issues are still being discussed. Traditional herbal medicines have arisen as effective agents to alleviate this multifactorial disease, and various studies have scrutinized the antiobesity effects of natural products. While many systemic reviews have examined the effects of natural products against obesity, none have systematically categorized natural drugs and mechanisms. In addition, this resviearchw is the most recent to assess extensive natural products. This researchview summarizes the effects and related mechanisms of each natural product studied in clinical trials. The natural products were classified into seven groups: natural compounds, foods, teas, fruits, extracts, decoctions, and external preparations. The mechanisms of the natural products were organized into lipid metabolism, anti-inflammation, antioxidant, appetite loss, and thermogenesis.
3. Discussion
4.1. Antiobesity Mechanism
3.1. Antiobesity Mechanism
Based on the reviewed studies, natural products that demonstrated efficacy in alleviating obesity shared common mechanisms. Major mechanisms included lipid metabolism, anti-inflammation, antioxidation, appetite loss, and thermogenesis. The efficacy was evident in regulating lipid parameters, cytokines, hormones, or genes. By comprehensively understanding the efficacy and related mechanisms, this re
svie
archw extensively identified the potential effects of various natural products for treating obesity.
34.1.1. Lipid Metabolism
Various studies identified lipid metabolism when discussing how the target compound works to treat obesity (
Figure 1 and
Figure 2). Forty-five studies were regarded to have a lipid metabolism pathway, although nine studies lacked an appropriate mechanism.
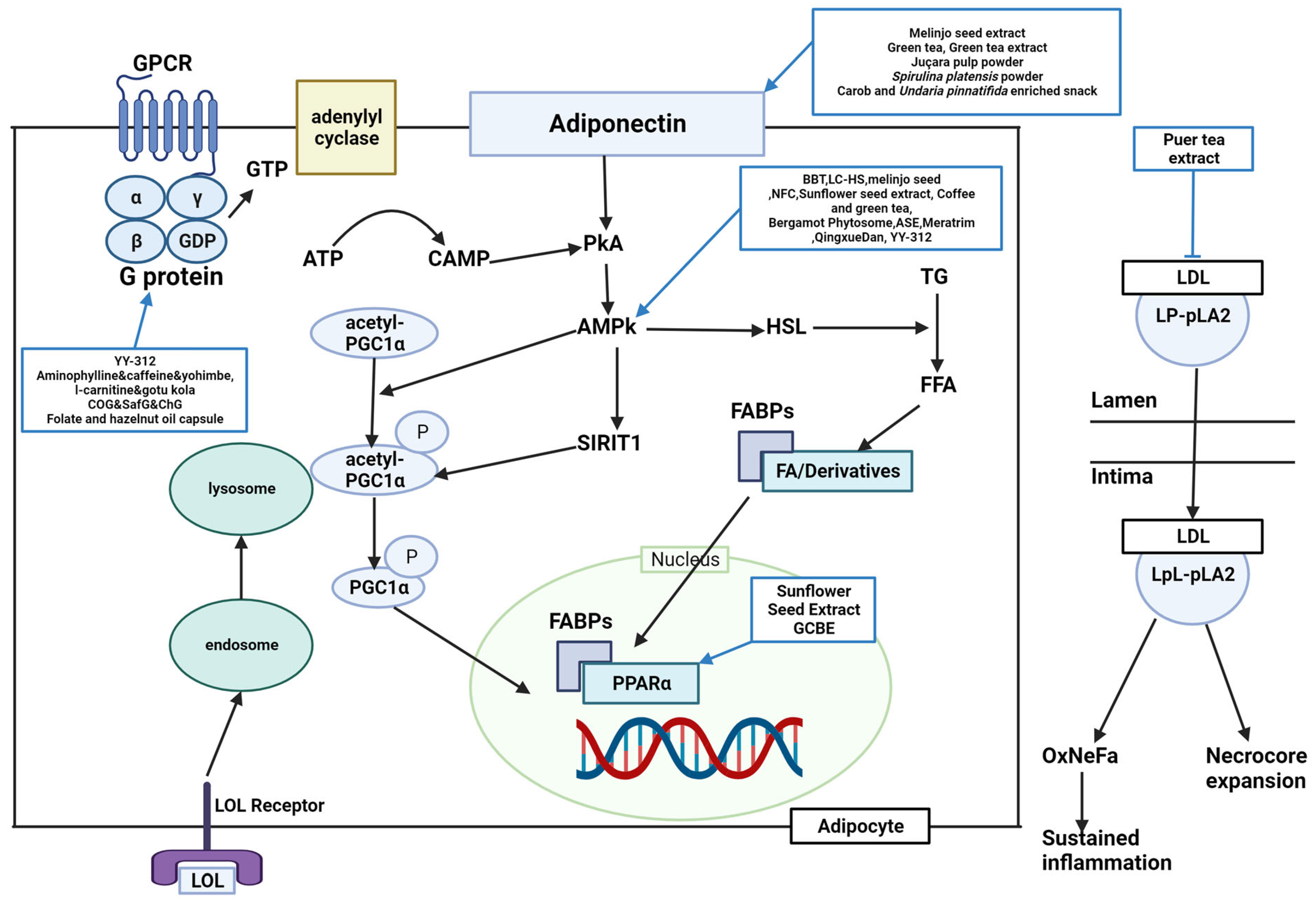
Figure 1. Schematic diagram of lipid metabolism and the effects of natural products. BBT, black soybean testa extract; LPL, lipoproteinlipase; HSL, hormone sensitive lipase; AMPK, adenosine monophosphate-activated protein kinase; NFC, Nigella sativa and Trigonella foenum graecum supplemented chapatis; LC-HS, Lippia citriodora L. and Hibiscus sabdariffa L; ASE, Aster spathulifolius Maxim extract; OPE, onion peel extract; CPT-1α, carnitine palmitoyltransferase I alpha; GCBE, green coffee bean extract; YY-312, Imperata cylindrica Beauvois, Citrus unshiu Markovich, and Evodia officinalis Dode; PPARα, peroxisome proliferator-activated receptor alpha; Lp-PLA2, lipoprotein-associated phospholipase A2; LDL, low-density lipoprotein; CoG, coconut oil group; SafG, safflower oil group; ChG, chia oil Group; GTP, guanosine triphosphate; ADRB3, A/T-, and β-adrenergic receptor 3; GPCR, G protein-coupled receptor; GTP, guanosine triphosphate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; AMPK, adenosine monophosphate-activated protein kinase; TG, triglycerides; FFA, free fatty acids; FABPs, fatty acid-binding protein; FA, fatty acid; OxNeFa, oxidized nonesterified fatty acids; PGC1α, peroxisome proliferator-activated receptor-gamma coactivator-1 alpha; PKA, protein kinase A; LpL-PLA2, lysophospholipid-associated phospholipase A2.
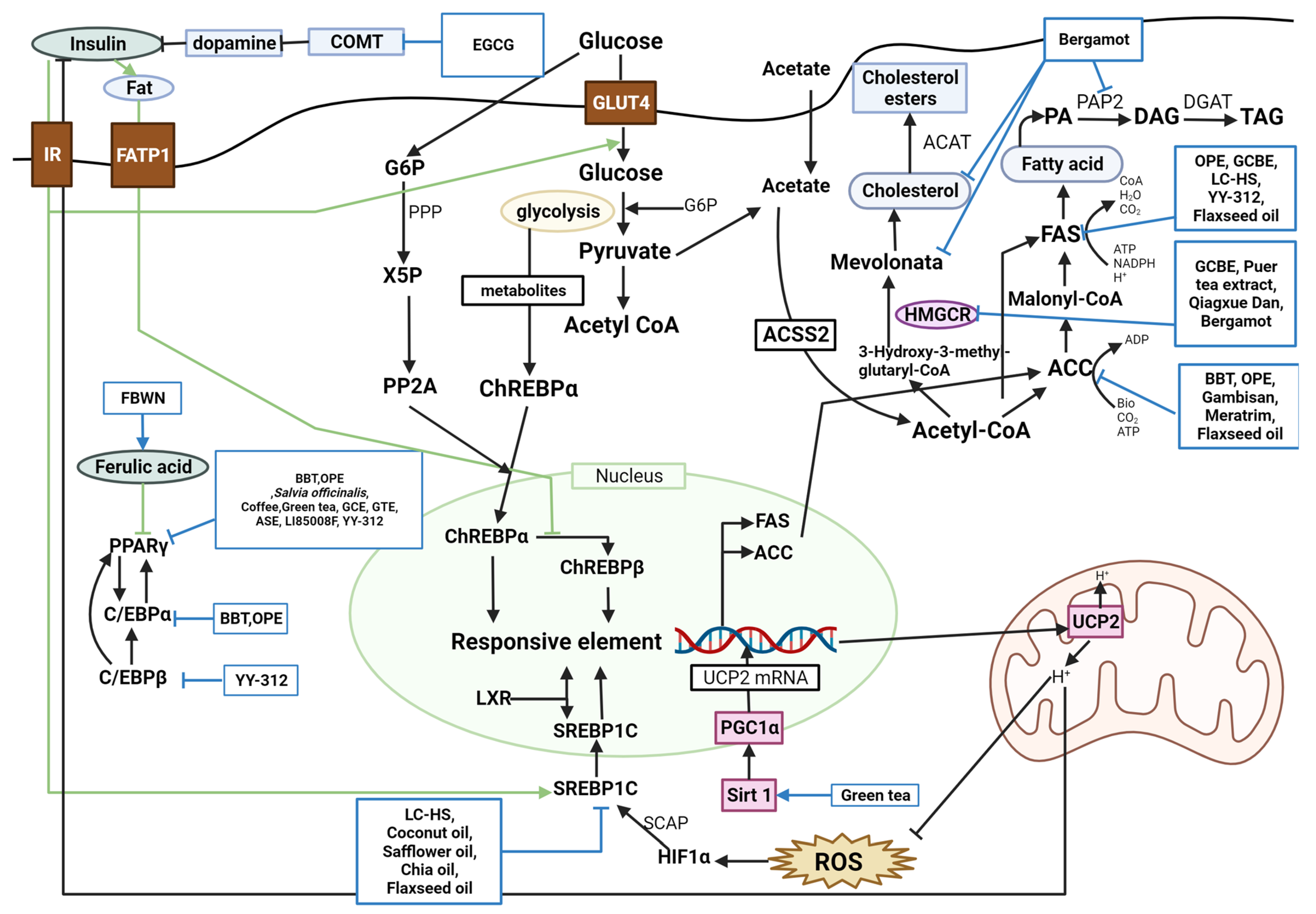

Figure 2. Schematic diagram of glucose metabolism and the effects of natural products. PPARγ, peroxisome proliferator-activated receptor gamma; BBT, black soybean testa extract; OPE, onion peel extract; GCBE, green coffee bean extract; GTE, green tea extract; ASE, Aster spathulifolius Maxim extract; LI85008F, Moringa oleifera leaf aqueous ethanol extract, Murraya koenigii (L.) Spreng. leaf aqueous ethanol extract, and Curcuma longa L. extract; YY-312, Imperata cylindrica Beauvois, Citrus unshiu Markovich, and Evodia officinalis Dode; ACC, acetyl-CoA carboxylase; ATP, adenosine triphosphate; ADP, adenosine diphosphate; C/EBPα, CCAAT-enhancer-binding protein alpha; FAS, fatty acid synthase; PA, phosphatidic acid; PAP2, type-2 phosphatidic acid phosphatase; DAG, diacylglycerol; DGAT, diacylglycerol-acyltransferase; TAG, triacylglycerol; LC-HS, Lippia citriodora L. and Hibiscus sabdariffa L.; NADPH, nicotinamide adenine dinucleotide phosphate; CoA, coenzyme A; C/EBPβ, CCAAT/enhancer-binding protein beta; HMGCR, HMG-CoA reductase; ACAT, acylCoA cholesterol acyl transferase; srebp-1c, sterol regulatory element-binding protein 1c; LXR, liver X receptor; FBWN, Lactobacillus plantarum fermented barley-wheat flour compound noodle; COMT, catechol-O-methyltransferase; IR, insulin receptor; FATP1, fatty acid transport protein 1; GLUT4, glucose transporter type 4; G6P, glucose-6-phosphate; Acetyl CoA, acetyl coenzyme A; ACSS2, acetyl-CoA synthetase 2; ChREBPα, carbohydrate response element binding protein α; ChREBPβ, carbohydrate response element binding protein β; UCP2, uncoupling protein 2; ROS, reactive oxygen species; HIF1α, hypoxia inducible factor 1; SCAP, stem cells from apical papilla; SIRT1, sirtuin-1; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; UCP2 mRNA, uncoupling protein 2 messenger RNA; PPP, phosphatidate phosphohydrolase; X5P, xylulose 5-phosphate; PP2A, protein phosphatase 2A.
Lipid metabolism is classified into lipogenesis, lipolysis, and adipocyte differentiation, and the corroborated antiobesity effects are explained by suppressing lipogenesis, accumulation, and adipocyte differentiation and inducing lipolysis and fatty acid oxidation. Peroxisome proliferator-activated receptor gamma (PPARγ), acetyl-CoA carboxylase (ACC), CCAAT-enhancer-binding protein alpha (C/EBPα), CCAAT/enhancer-binding protein beta (C/EBPβ), fatty acid synthase (FAS), and sterol regulatory element-binding protein 1c (SREBP-1C) are lipogenic factors. Lipoprotein lipase (LPL), hormone-sensitive lipase (HSL), peroxisome proliferator-activated receptor alpha (PPARα), and adenosine monophosphate (AMP)-activated protein kinase (AMPK) are lipolysis factors. Several studies found these factors to regulate obesity complexly.
PPARγ was downregulated by BBT, OPE, common sage, coffee, green tea, GCBE, GTE, ASE, LI85008F, and YY-312
[11][22][25][35][39][41][54][55][56][11,22,25,35,39,41,57,61,64]. BBT, OPE, Gambisan, Meratrim, and flaxseed oil are associated with ACC inhibition
[11][22][57][58][59][11,22,63,67,71]. BBT and OPE have been reported to downregulate C/EBPα
[11][22][11,22]. FAS was inhibited by OPE, GCBE, LC-HS, YY-312, and flaxseed oil
[22][40][55][59][60][22,40,61,62,71]. YY-312 downregulated C/EBPβ, thereby inhibiting adipocyte differentiation
[55][61]. BBT upregulated lipolysis proteins such as LPL and HSL
[11]. AMPK is predominantly associated with antiobesity metabolism. BBT, LC-HS, melinjo seed, NFC, sunflower seed extract, coffee and green tea, bergamot phytosome, ASE, LC-HS, Meratrim, Qingxue Dan, and YY-312 were reported to activate AMPK
[11][18][20][21][28][35][47][54][55][57][60][61][11,18,20,21,28,35,47,57,61,62,63,65]. OPE upregulated carnitine palmitoyltransferase I alpha (CPT-1α)
[22]. Sunflower seed extract and GCBE upregulated
PPARα expression
[28][40][28,40]. APN was upregulated by a carob- and wakame-enriched snack, melinjo seed, green tea, GTE, juçara pulp powder, and
S. platensis powder, decreasing lipogenesis and inducing β-oxidation
[13][20][36][41][51][62][13,20,36,41,51,54]. Pancreatic lipase was inhibited by PTE, mangosteen extract, Gambisan, and Qingxue Dan
[45][52][58][61][45,52,65,67]. HMG-CoA reductase (HMGCR), a cholesterol synthesis enzyme, was inhibited by GCBE, PTE, and Qingxue Dan
[40][45][61][40,45,65]. PTE acts as a noncompetitive inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2)
[45]. LC-HS, coconut oil, safflower oil, chia oil, and flaxseed oil suppressed SREBP-1C
[59][60][63][62,71,74]. A carob- and wakame-enriched snack and Qingxue Dan activated the LDL receptor, inhibiting lipid synthesis
[13][61][13,65]. YY-312, Lipoxyderm, coconut oil, safflower oil, chia oil, and folate and hazelnut oil capsules altered steps in the process in which activation of guanosine triphosphate (GTP)-binding proteins successively activates adenylate cyclase, cyclic AMP (cAMP), and protein kinase A (PKA), and lipase
[55][63][64][65][61,69,72,74]. GCBE and bergamot inhibited the activation of acyl-CoA cholesterol acyl transferase (ACAT)
[40][49][40,49]. GCBE upregulated carnitine palmitoyl transferase, a fatty acid oxidation enzyme
[40]. FBWN increased ferulic acid, inhibiting lipid accumulation and regulating lipid metabolism
[17]. High-dose GTE increased fat oxidation by inhibiting catechol-O-methyltransferase (COMT)
[42]. Quercetin-rich onion powder altered the expression of genes related to fat metabolism, such as ADRB3, HSL, PPARγ, and uncoupling protein (UCP)-2
[24]. Standardized extracts of Malabar tamarind konjac regulated lipolysis by activating catecholamine signaling
[26]. Green tea increased SIRT1-mediated PGC-1α activity, decreasing adipocyte differentiation, proliferation, and the expression of genes involved in lipogenesis
[36]. Bergamot inhibited HMGCR and reduced cholesterol levels, mevalonate levels, and hepatic TG accumulation by inhibiting phosphatidate phosphohydrolase activity
[49].
34.1.2. Anti-Inflammation
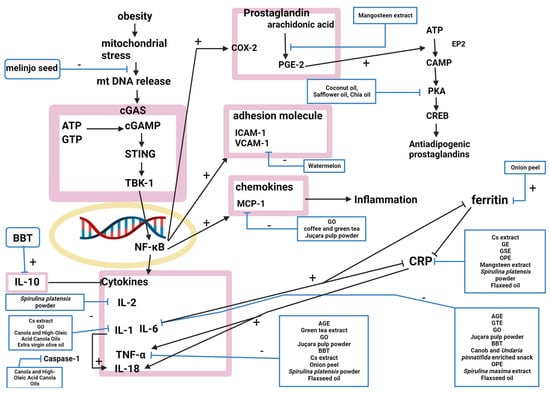
Twenty-one natural products modulated the inflammation pathway, and twenty studies explained their mechanisms (
Figure 3).
Figure 3. The nuclear factor-kappa B (NF-κB) signaling pathway was inhibited by AGE, GTE, GO, and juçara pulp powder, attenuating the production of proinflammatory cytokines and suppressing obesity-induced inflammation
[9][41][50][51][9,41,50,51]. NF-κB inhibition decreases the circulating levels of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin (IL)-6. IL-6 was decreased by AGE, BBT, a carob- and wakame-enriched snack, OPE, GTE, GO, juçara pulp powder,
S. maxima extract, and flaxseed oil
[9][11][13][22][41][50][51][59][66][9,11,13,22,41,50,51,53,71]. TNF-α was inhibited by AGE, BBT, artichoke extract, GSE, onion peel, GTE, GO, juçara pulp powder,
S. platensis powder, and flaxseed oil
[9][11][14][16][22][41][50][51][59][62][9,11,14,16,22,41,50,51,54,71]. Coffee and green tea, GO, and juçara pulp powder downregulated the expression of monocyte chemoattractant protein-1 (MCP-1), inhibiting monocyte adhesion
[35][50][51][35,50,51]. Artichoke extract, GE, GSE, OPE, mangosteen extract,
S. platensispowder, and flaxseed oil decreased high-sensitivity C-reactive protein (hsCRP) levels
[14][15][16][22][52][59][62][14,15,16,22,52,54,71]. GO, canola oil, high-oleic-acid canola oil, and extra virgin olive oil reduced IL-1β
[50][67][68][50,70,73]. BBT increased IL-10
[11]. Artichoke extract regulated IL-1 and interferon (IFN)
[14]. Melinjo seed activated the cGMP-AMP (cGAMP) synthase-cGAMP-stimulator of interferon genes pathway by activating disulfide bond A oxidoreductase-like protein (DSBA-L)
[20]. Onion peel decreased ferritin
[22]. Watermelon decreased VCAM-1, intercellular adhesion molecule 1 (ICAM-1), and P-selectin, which attracts immune cells to damaged areas of the endothelium
[29]. Mangosteen extract inhibited the conversion of arachidonic acid to prostaglandin E2 (PGE2) by altering cyclooxygenase (COX) and COX2 gene expression
[52]. IL-2 was decreased by
S. platensispowder
[62][54]. Canola oil and high-oleic-acid canola oils inhibited inflammasome-mediated caspase-1 (CASP1) activity
[68][73]. Coconut, safflower, and chia oils upregulated cAMP-dependent signaling pathways, which produce antiadipogenic prostaglandins that function in the adaptive reactions of cyclooxygenases
[63][74].
34.1.3. Antioxidant
The next mechanism associated with antiobesity is antioxidation (
Figure 4). Ten studies noted antioxidant effects, but only two discussed the antioxidant mechanism associated with their compound’s significant efficacy.
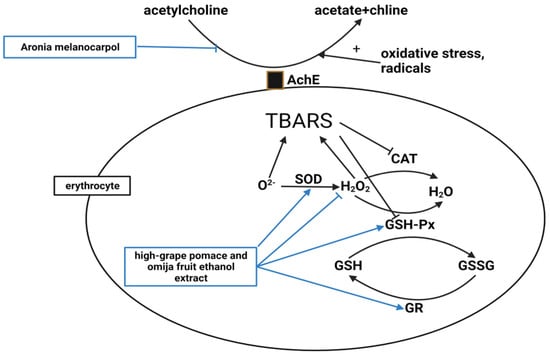
Figure 4. High oxidative stress and free radicals increase AChE activity. However,
A. melanocarpa decreased the AChE activity in the erythrocyte membranes
[46]. Superoxide dismutase (SOD) catalyzes the dismutation of the superoxide anion to H
2O
2, then catalase (CAT) and glutathione peroxidase (GSH-Px) degrade H
2O
2. GSH-Px also oxidizes reduced glutathione (GSH) to oxidized glutathione (GSSG), and GSSG is reduced to GSH by glutathione reductase (GR). A high-GO supplement elevated erythrocyte SOD, GSH-Px, and GR activities and lowered H
2O
2 levels
[50]. Thiobarbituric acid reactive substances (TBARS), a marker of lipid peroxidation caused by oxidative injury, were also reduced by the high-GO supplement
[50].
34.1.4. Appetite Loss
Twelve studies established a connection between appetite and the effects of natural products, and nine mentioned the mechanism underlying this effect (
Figure 5). Among the three excluded studies, one did not show a significant appetite suppression effect but reported a related mechanism. Appetite loss manifests as increased anorexigenic factors and decreased orexigenic factors.
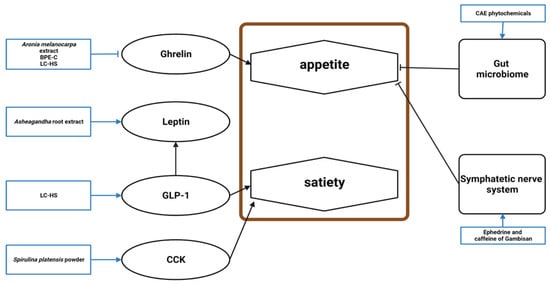
Figure 5. Schematic diagram of the appetite mechanism in obesity and the effects of natural products. BPE-C, bergamot polyphenol extract complex; LC-HS, Lippia citriodora L. and Hibiscus sabdariffa L.; GLP-1, glucagon-like peptide-1; CCK, cholecystokinin; CAE, caraway aqueous extract.
Ghrelin was lowered by
A. melanocarpa extract, BPE-C, and LC-HS
[46][48][60][46,48,62]. LC-HS increased glucagon-like peptide-1 (GLP-1), an anorexigenic incretin produced by the intestinal L-cells that stimulates insulin secretion and induces satiety
[18][60][18,62]. Ashwagandha root extract reduced stress, restoring leptin levels, which suppresses food intake
[69][56]. CAE phytochemicals, including limonene, γ-terpinene, trans-carveol, carvone, thymol, and carvacrol, improved the gastrointestinal microbiome to alter appetite
[12]. The phenylalanine content of
S. platensis powder may be responsible for cholecystokinin release, which affects the brain’s appetite center
[62][54]. The ephedrine and caffeine in Gambisan reversed obesity by reducing food intake via the sympathetic nervous system
[58][67].
34.1.5. Thermogenesis
Ten studies mentioned the relationship between thermogenesis and the effects of natural products, though one only stated the effects without explaining the mechanism (
Figure 6). Increased thermogenic gene expression and factors caused the browning of white adipose tissues.
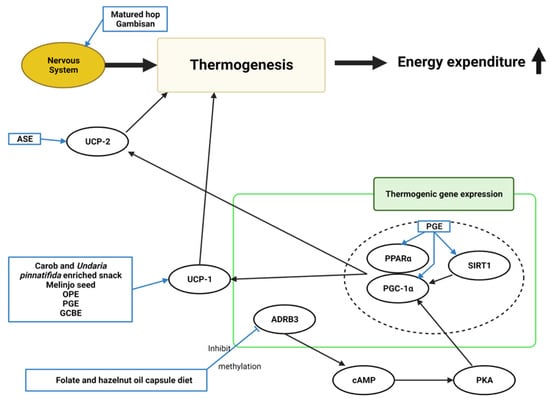
Figure 6. Schematic diagram of the thermogenesis mechanism in obesity and the effects of natural products. ASE, Aster spathulifolius Maxim extract; OPE, onion peel extract; PGE, Platycodon grandiflorusethanol extract; GCBE, green coffee bean extract; UCP-1, uncoupling protein-1; UCP-2, uncoupling protein-2; ADRB3, adrenoceptor beta-3; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; PPARα, peroxisome proliferator-activated receptor alpha; SIRT1, sirtuin 1; ↑, increase.
A carob- and wakame-enriched snack, melinjo seed, OPE, PGE, and GCBE induced uncoupling protein-1 (
UCP1) in brown adipose
[13][20][22][23][40][13,20,22,23,40]. ASE increased uncoupling protein-2 (
UCP2) expression, increasing energy expenditure and consumption
[54][57]. The sympathetic nervous system was considered related to energy expenditure through thermogenesis. Matured hop and Gambisan were believed to activate the nerve system
[19][58][19,67]. PGE increased the expression of thermogenic-related genes, such as
SIRT1,
PPARα, and
PGC-1α [23]. Folate and hazelnut oil capsules lowered
ADRB3 gene methylation levels
[65][72]. The ADRB3 protein facilitates the catecholamine-induced activation of adenylate cyclase through the actions of G proteins. These mechanisms are involved in energy homeostasis by mediating thermogenesis.
It is evident that various studies have examined the effects of natural products on obesity. This resviearchw detailed the potential for the widespread use of natural products in treating obesity, which has not been reported in previous reviews on the same topic. FBased on this review, further studies on safety, tolerability, and pharmacokinetics can be performed on these natural products to confirm their potential effectiveness. Natural compounds, foods, tea, fruit, extracts, decoctions, and external preparations were found to show efficacy in lipid metabolism, anti-inflammation, antioxidation, appetite loss, and thermogenesis. Most studies showed positive effects in relieving the symptoms of obesity and demonstrated that natural products could be used as effective treatments for obesity. Therefore, herbal medicines are expected to be fully utilized in clinical obesity treatment. However, limitations remain in that some studies did not investigate efficacy or safety, and their nonsignificant results could be changed with precise control of drug dosages. Therefore, meta-analyses are needed to further examine their findings. Further studies are expected to refine the pharmacological effects of natural products for clinical use.