Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vyacheslav Arlyapov and Version 2 by Camila Xu.
Conductive polymers (CPs) are a class of organic compounds that belong to a large group of electrochemically active polymers. They have been actively studied for more than 40 years, beginning with the discovery of the conductive properties of halogen-substituted polyacetylene and the preparation of polypyrrole.
- conducting polymers
- nanocomposites
- biosensors
- microbial and enzymatic biofuel cells
1. Introduction
Conductive polymers (CPs) are a class of organic compounds that belong to a large group of electrochemically active polymers [1]. They have been actively studied for more than 40 years, beginning with the discovery of the conductive properties of halogen-substituted polyacetylene [2] and the preparation of polypyrrole [3]. CPs are an important class of functional materials that have found wide application in many areas of science [4]. They have excellent properties, such as adjustable conductivity, ease of application to the electrode surface, and the possibility of simple chemical modification. Due to their biocompatibility, they are used in the field of regenerative medicine [5][6][5,6], tissue engineering [7][8][7,8], electrically induced targeted drug release systems [9][10][9,10], and cell cultivation [11]. It is also possible to use conductive materials in energy storage devices, electronic devices, and anticorrosion coatings [12][13][14][15][16][17][12,13,14,15,16,17]. Due to their high conductivity, such polymers and composites based on them are excellent materials for the conjugation of biological materials and electrodes in bioelectrochemical systems [18][19][20][18,19,20]. At the same time, conductive polymers can act as effective matrices for the immobilization of biological materials, enabling the free diffusion of substrates and reaction products and providing a biocompatible environment for enzymes or microorganism cells (Figure 1).
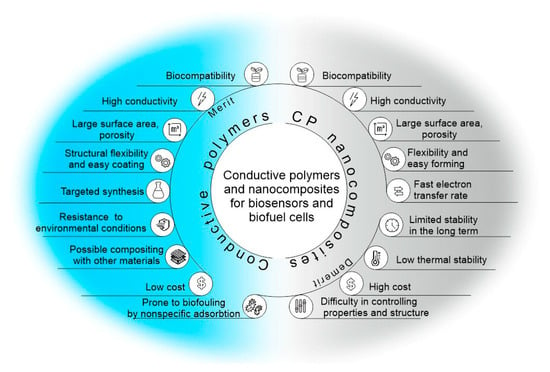
Figure 1.
Advantages and disadvantages of conducting polymers for use in biosensors and biofuel cells.
Electrically conductive polymeric materials are divided into two large groups. The first group includes polymers with ionic conductivity, or solid polymer electrolytes, and the second group includes polymers with electronic conductivity. In turn, polymers with electronic conductivity are divided into so-called organic metals (polymers with a conductivity similar in mechanism to the electrical conductivity of metals and called “conducting polymers”) and redox polymers (compounds in which electron transfer is carried out mainly due to the flow of oxidative–reduction reactions between neighboring fragments of the polymer chain) [21].
The electronic conductivity of conducting polymers exists due to delocalized electrons (n-conductivity) or holes (p-conductivity) (a unit charge in such polymers, as a rule, is delocalized over several fragments of the polymer chain). The conductivity of conducting polymers is due to the generation of charge carriers (polarons and bipolarons) in the polymer phase as a result of the oxidation of double bonds and heteroatoms with unshared electron pairs [4]. Polarons and bipolarons are radical cations and dications delocalized in the polymer chain. The delocalization of the charge and unpaired electrons in polarons and bipolarons leads to the structural deformation of the polymer lattice, while the stabilization of polaron charge carriers occurs due to polarization and interaction with counterions. During the migration of polarons and bipolarons along the polymer chain, the electronic conductivity of polymers is realized by the reorganization of single and double bonds. In defective areas, charge transfer is carried out due to interchain activation “jumps” of electrons, that is, the “hopping mechanism”.
Redox-active polymers are also a class of electrochemically active compounds; however, they differ due to the presence of discrete redox-active centers [22]. The process of electron transfer in the polymer layer can be represented as a sequence of reactions involving the self-exchange of electrons between redox centers in different oxidation states. The conductivity of the polymer reaches its maximum value when the volume concentrations of oxidized and reduced fragments are equal, namely, at the formal potential of the redox system. Both redox-active and conductive ones have a mixed electronic–ionic conduction mechanism, which includes the interfacial transfer of electrons and ions through the phase boundaries and the conjugate electron–ion transfer in the bulk of the material. The charge transfer in the electrically conductive layer is accompanied by the diffusion of supporting electrolyte ions into the bulk of the polymer to maintain its electrical neutrality. To describe the charge transfer in redox-active and conducting polymers, the same model of the “hopping mechanism” accompanied by ion transfer is used [4]. Polymerized phenazines (poly(thionine), poly(neutral red), poly(methylene blue), etc.) occupy a special place in the group of electron-conducting polymers. These phenazines have delocalized π electrons in their structure, like conducting polymers; however, during polymerization, they retain electron transfer in separate monomeric fragments, and, therefore, they are commonly referred to as redox-active polymers [23][24][25][26][23,24,25,26].
The electrical properties of conducting polymers can be varied by doping, increasing the degree of charge carriers [27], which, in turn, will increase their applications, particularly in bioanalytical and bioelectrochemical devices. In the undoped state, conducting polymers behave as anisotropic quasi-one-dimensional electronic structures with a moderate bandgap of 2–3 eV, as in a conventional semiconductor. When a conductive polymer is doped or photoexcited, the polymer changes to a “metallic” state. In recent decades, nanomaterials have been the most commonly used dopants due to their excellent electrocatalytic properties, multiple active sites, structural properties, and good stability. Such nanomaterials can also be effectively used in combination with conductive polymers in the modification of biosensor electrodes or BFCs. Simplification of the conditions for the synthesis of various nanomaterials has led to an increase in the number of works in the field of nanocomposites with conductive polymers in the last 10 years (Figure 2).
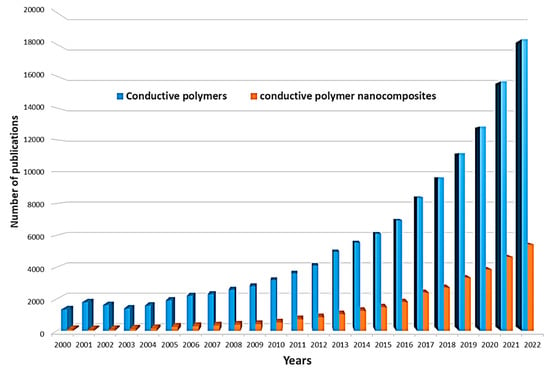
Figure 2. Number of publications in the ScienceDirect database per year with conductive polymers and conductive polymer nanocomposite as keywords.
2. Synthesis of Conducting Polymers
The most widely used conductive polymers are polyacetylene, polyaniline, polypyrrole, polythiophene, poly(para-phenylene), poly(phenylvinylene), and polyfuran (Figure 3). Their electrical properties are presented in Table 1.
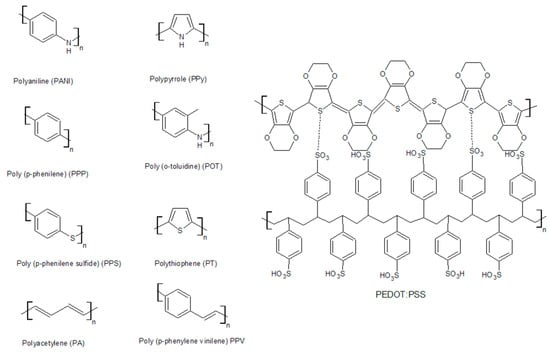
Figure 3.
Structural forms of the most common conductive polymers.
| Conductive Polymer | First Synthesized | Molecular Weight of the Monomer (g mol−1) |
Conductivity Type | Specific Capacitance (Fg−1) | Conductivity (S cm−1) |
|---|---|---|---|---|---|
| Polyacetylene | 1977 | 26 | n, p | 241 | 103–1.7 × 105 |
| Polypyrrole | 1979 | 67 | P | 530 | 102–7.5 × 103 |
| Polyparaphenylene | 1979 | 78 | n, p | - | 102–103 |
| Polyparavinylene | 1979 | 28 | P | - | 3–5 × 103 |
| Poly(3,4-ethylenedioxythiophene) | 1980 | 142 | n, p | 92 | 300 |
| Polyaniline | 1980 | 93 | n, p | 240 | 30–200 |
| Polythiophene | 1981 | 84 | p | 485 | 10–103 |
One of the most successful polymers in terms of synthesis is PEDOT:PSS (the synthesis is patented by Bayer). Its production reaches more than 100 tons per year. The production of polypyrrole and polyaniline is also widely scaled [31]. Conductive polymers are obtained in several ways, the main one of which is polymerization. Polymerization consists of three main steps: oxidation, binding, and deprotonation.
The process begins with the oxidation of monomer units to form radical cations, which then combine with each other or with other monomers. Bound cations are deprotonated, and dimer fragments are formed [32][33][32,33]. The process of oxidation and binding is repeated with dimers, leading to an increase in the chain and the formation of long chains of polymers [15]. There are several ways to initiate polymerization, such as chemical, electrochemical, and photopolymerization.
2.1. Chemical Synthesis of Conductive Polymers
Chemical polymerization is one of the most widely used methods for producing conductive polymers, along with electropolymerization. The method is scalable and economical and does not require expensive equipment. Usually, in chemical synthesis, the polymer has fewer internal crosslinks between macromolecules, and therefore, it is more soluble in suitable solvents. In addition, this method has more opportunities for the covalent modification of conducting polymers [34]. However, chemical polymerization does not always ensure the high electrical conductivity of the obtained polymers, does not allow for controlling the thickness of coatings, and is more suitable for obtaining thick-film and powder polymers. Also, oxidizing agents are required for the synthesis by this method, which can lead to contamination of the final product [35].
Chemical polymerization is carried out using various oxidizing agents, such as ammonium persulfate ((NH4)2S2O8), sodium vanadate (NaVO3), cerium sulfate (Ce(SO4)2), hydrogen peroxide (H2O2), potassium iodate (KIO3), potassium dichromate (K2Cr2O7), and hydrochloric acid (HCl4) [36][37][38][39][36,37,38,39]. The mechanism of chemical polymerization, using polypyrrole as an example.
The use of the catalytic action of redox enzymes makes it possible to carry out chemical polymerization under milder and environmentally friendly conditions. For example, peroxidase and laccase are used to synthesize polyaniline and polypyrrole [40][41]. The review in [41][42] considers the latest achievements over the last 10 years in the synthesis of polyaniline using laccase with atmospheric oxygen, and it is shown that this synthesis method is variable in terms of settings that can be controlled using templates.
Interfacial polymerization is an example of chemical oxidative polymerization that has found wide application in the preparation of conductive polymers for chemical sensors, fuel cells, supercapacitors, and membranes with selective permeability. This method includes several types of polymerization with phase separations: liquid–solid, liquid–liquid, and liquid-in-liquid.
Reference [42][44] reports that PEDOT obtained by interfacial polymerization acquires unique properties, such as a high porosity of 70.61%, a high specific surface area > 58 m2/g, and an ideal electrical conductivity of 6500 S/m, which provides a wide voltage range for supercapacitors (up to 1.2 V). The material demonstrates outstanding energy storage characteristics in electronics, while the proposed method is environmentally friendly.
2.2. Electropolymerization of Conductive Polymers
Electrochemical polymerization is the most commonly used method for the synthesis of conductive polymers because it allows better control of polymer deposition, film morphology, electrical conductivity, and mechanical properties. In addition, this method allows the direct application of the polymer to the substrate/substrate. This method is not suitable for all types of conductive polymers. In addition, it is difficult to scale and can lead to the irreversible oxidation of monomers due to the application of high potentials [34]. Electrochemical polymerization is carried out using an electrochemical cell with a three-electrode system containing working, counter, and reference electrodes. The solution typically contains monomers, a solvent, and an electrolyte/salt. Polymerization can be initiated using cyclic voltammetry, potentiostatically, or using a galvanostatic method. The potentiostatic method makes it easy to control the film thickness using Faraday’s law, while potentiodynamic methods lead to the formation of more uniform and adhesive films on the electrode. The galvanostatic method is generally considered more efficient because it allows the growth of the conductive polymer film to be monitored and the change in conductivity during the polymerization process.
The method is widely used for the synthesis of many known conductive polymers, for example, polyaniline [48][50], polypyrrole [49][50][51,52], and PEDOT [37].
2.3. Photochemical Polymerization of Conductive Polymers
Photopolymerization is a special form of radical polymerization that uses light to initiate polymerization. Gamma rays [51][53], microwaves [52][54], ultraviolet rays [53][55], and X-rays [53][55] are used as sources of radiation. Moreover, the synthesis of conductive polymers proceeds indirectly using photosensitizers. In this case, the transfer of light energy occurs through a photosensitizer to form the corresponding excited states. Ruthenium complexes, silver nitrate, camphorquinone, and ketones are used as photosensitizers. For photoelectrochemical polymerization, the photosensitizer is a dye-sensitized semiconductor (metal oxides such as TiO2, ZnO, and WO3; chalcogenides such as CdS, CdSe, and GaAs) or simply a dye.
Photochemical polymerization takes place at lower temperatures than chemical polymerization and allows the control of the reaction by simply turning on/off the light source [53][54][55,56]. This method solves the problem of excessive oxidation of the obtained polymers and does not harm the environment; however, not all polymers can be obtained by this method [28].
The main limitation of light-induced polymerization is the insignificant depth of light penetration, which does not exceed a few millimeters and depends on the wavelength and spectral distribution. Photopolymerization techniques are only applicable to thin-film applications because polymerization stops when the light source is removed. This method is used for the synthesis of polypyrrole [55][57], polyaniline, and hybrid materials based on polyaniline [56][58], as well as some other conductive polymers.
2.4. Synthesis of Conductive Nanocomposites
The use of pure polymers is limited; therefore, their composites with various compounds are obtained. For example, the synthesis of nanocomposites can occur in situ (sequentially), ex situ (separately), and in one pot (simultaneously). Approaches to the synthesis of nanocomposites can be divided into synthesis using templates, template-free synthesis, and other methods. They are described in detail in [28][34][57][28,34,59].
The template method involves applying hard or soft templates. Solid templates are used as membranes to obtain nanostructures inside the pores or channels of the membrane to control the morphology of the future polymer. Carbon nanotubes, graphite, aluminum oxide, and vanadium are described as templates. The synthesis of nanopolymers is carried out chemically and electrochemically. The disadvantages of this approach include the fact that it is limited in terms of production scale, templates are needed for synthesis, and the use of aggressive reagents is required to remove templates after the reaction.
The solution to these problems can be a soft template method based on the use of surfactants, micelles, and structure-forming molecules, which are usually created using self-assembly mechanisms using hydrogen bonds, etc. The morphology of the obtained conducting polymers directly depends on the morphology and molecular matrices of the templates. The method is distinguished by its simplicity, its low cost, and the ability to carry out synthesis on a large scale. However, the disadvantages include the difficulty of controlling the size and orientation of the conductive structures. This method is used for the synthesis of polyaniline [58][61]. The article considers the influence of anionic polyelectrolytes, micelles, and vesicles as matrices for obtaining conductive polymers. It has been shown that obtaining polyaniline with a higher content of the polaron form is observed when using vesicles from sodium bis(2-ethylhexyl)sulfosuccinate as a matrix.
The templateless method allows one to bypass the limitations of the templated method. In this case, combined materials are used to obtain a highly organized architecture of finished products. Synthesis is controlled by varying the temperature and the ratio of the monomer and dopant. Commonly used zero-template technologies include electrospinning, seeding, interfacial polymerization (which is described above), and other nanofabrication methods [52][54].
Electrospinning is a universal method that allows for obtaining ultrathin fibers as a result of the action of electrostatic forces on an electrically charged jet of a polymer solution or melt. The electrospinning method makes it possible to obtain polymer fibers with a diameter of several hundred nanometers. The formation of ultrathin fibers by electrospinning is carried out by the uniaxial electrical stretching of a viscoelastic solution. Electrospinning requires three main components: a high-voltage power supply, a solution container with a spinneret, and a grounded metal collector [59][62].
The study in [60][63] describes a one-step method of polypyrrole polymerization and in situ immobilization in a poly(ε-caprolactone) polymer matrix to develop an efficient conductive biomaterial that has a potential result for bone tissue engineering. The increased surface-area-to-volume ratio due to the fine nanofiber morphology, the conductive properties of the highly conjugated PPy backbones, and improved mechanical strength, surface wettability, and biological activity contributed to better cell attachment and proliferation in PCL/PPy conductive scaffolds.
Comparing all of the methods for the synthesis of conductive polymers and their nanocomposites discussed above, it is obvious that the possibilities for the synthesis of conductive polymers and nanocomposites are enormous. Strategies for integrating nanostructures into a polymer matrix with the desired strong interfacial interactions have further advanced this unique group of materials that will find wide application. At the same time, the possibilities for synthesis may develop toward the appearance of new monomers with higher conductivity. An increasingly important role in the synthesis of nanocomposites will be played by a controlled nanoarchitecture, which will allow the structures and properties of future nanocomposites to be set with high accuracy, as well as the inclusion of ionic liquids that improve the synthesis process and the quality of the materials obtained. The movement of synthesis toward greater environmental friendliness will be quite relevant: the use of biological templates and the use of methods with a lower load on the environment. These trends in the synthesis of conductive polymers and their nanocomposites make it possible to obtain more efficient and functional materials with a wide range of capabilities in various fields, including electronics, energy, medicine, sensorics, and others.
