You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Xiaoyong Jia.
Photo-controlled self-assembly of nanoparticles (NPs) is an advanced and promising approach to address a series of material issues from the molecular level to the nano/micro scale, owing to the fact that light stimulus is typically precise and rapid, and can provide contactless spatial and temporal control. Photoexcitation-induced assembly of NPs is another promising physical strategy, and such a strategy aims to employ molecular conformational change in the excited state (rather than the chemical structure) to drive molecular motion and assembly.
- photo controlled
- nanoparticles
- self-assembly
- photoresponsive molecules
1. Photophysical Process
Photophysical processes are environmentally friendly and easy to control, and have attracted extensive research attention in recent years. In particular, photophysical processes based on spatial conformational changes to excited states have unique advantages. First, a molecule can quickly change from the ground to excited states under light excitation, and this change can be quickly recovered when the excitation source is removed, providing a basis for realizing real-time and in situ regulation. Second, changes in the spatial conformation between the ground and excited states of the molecule belong to a photophysical process, and the molecular structure remains unchanged. This prevents the formation of by-products and the problem of incomplete reaction conversion, which can effectively reduce the existence of assembly defects. Finally, the entire process is based on the excited state conformational change, which does not require the introduction of traditional photoresponsive molecules; thus, it is more conducive to the development of molecular systems. More attention has been paid to the regulation of molecular structures and properties in the excited state, and some material systems based on conformational changes in the excited state have been developed [77,78,79,80][1][2][3][4]. Up to now, photo-controlled crystal growth, photo-regulated luminescence, and photo-controlled aggregation have been realized in multiple molecular systems by tuning the excited state conformation [81,82,83][5][6][7]. Among these, the most systematic research has been conducted on the photo-controlled behavior of polyphenylthiobenzene molecules.
2. Photo-Controlled Behavior Polyphenylthiobenzene Derivatives
Previous studies have demonstrated that polyphenylthiobenzene derivatives have rich and easily controlled optical properties, as well as molecular crystals under different self-assembly driving forces. Therefore, these compounds have various external stimuli (light, pH, ion, force, and solvent)-responsive behavior, exhibiting reversible changes in optical properties and molecular configurations under stimuli [84,85,86,87,88,89][8][9][10][11][12][13]. Polyphenylthiobenzene derivatives are a type of “star-shaped” twisted molecules with flexible and rotatable C–S–C bonds, which is conducive of the obvious difference between their molecular excited and ground state conformations. The multiple non-covalent interactions (including C–H … π, π … π, S–S, and hydrogen halide bonds) formed between molecules can further drive self-assembly. Moreover, the heavy-atom effect of polysulfide endows these molecules with a long-lived excited state, which guarantees the aggregation and self-assembly of excited molecules through motion and collision on a time scale. Based on these characteristics of polyphenylthiobenzene molecules, photo-controlled molecular aggregation in solution, photo-controlled crystal growth and realignment, photo-induced block copolymer directed self-assembly in organic phases, photo-regulated microphase separation–recognized circularly polarized luminescence, and photo-tuning phase transformation have been achieved [90,91,92][14][15][16].
2.1. Photo-Controlled Molecular Aggregation
Zhu et al. designed a polyphenylthiobenzene molecule (compound 1, Figure 71a) that can effectively dissolve in aqueous solutions [93][17]. Theoretical calculations indicate that the equilibrium geometry of the ground and excited states of this molecule differ significantly, which plays a decisive role in achieving photo-controlled molecular aggregation. In the ground state, torsion 1 = 120°, whereas torsion 1 = 90° in the excited state. During the transition from ground to excited states, the conformation of compound 1 changes significantly, and the structure of the excited state is significantly more regular than that of the ground state. As a result, the π···π interactions among the excited state molecules tend to be strengthened, leading to the molecules being parallel to each other and forming ordered stacking. Another key factor driving molecular aggregation is the transition of molecular hydrophilicity and hydrophobicity. The space between the molecular side chains in the ground state structure is calculated as 5.0 Å and is more compatible with H2O molecules (4.0 Å, Figure 71b). In this case, compound 1 behaves as hydrophilic molecules. The molecules in the ground state can be well dispersed in water, with a particle size not exceeding 10 nm. However, the space becomes smaller and is calculated as 4.1 Å in the excited state, which cannot be well matched with H2O molecules. In this case, compound 1 behaves as hydrophobic molecules. Based on these factors, compound 1 can form aggregates through photo irradiation. As the irradiation time increases, aggregates with a 200-nm diameter are formed (Figure 71c). These aggregates can disperse into aqueous solutions again when photo irradiation is removed because compound 1 recovers to be hydrophilic. The entire process of photo-controlled aggregation is a photophysical process based on the transformation of excited state conformation, which provides a reference for controlling excited state conformation to realize photo-controlled molecular self-assembly.
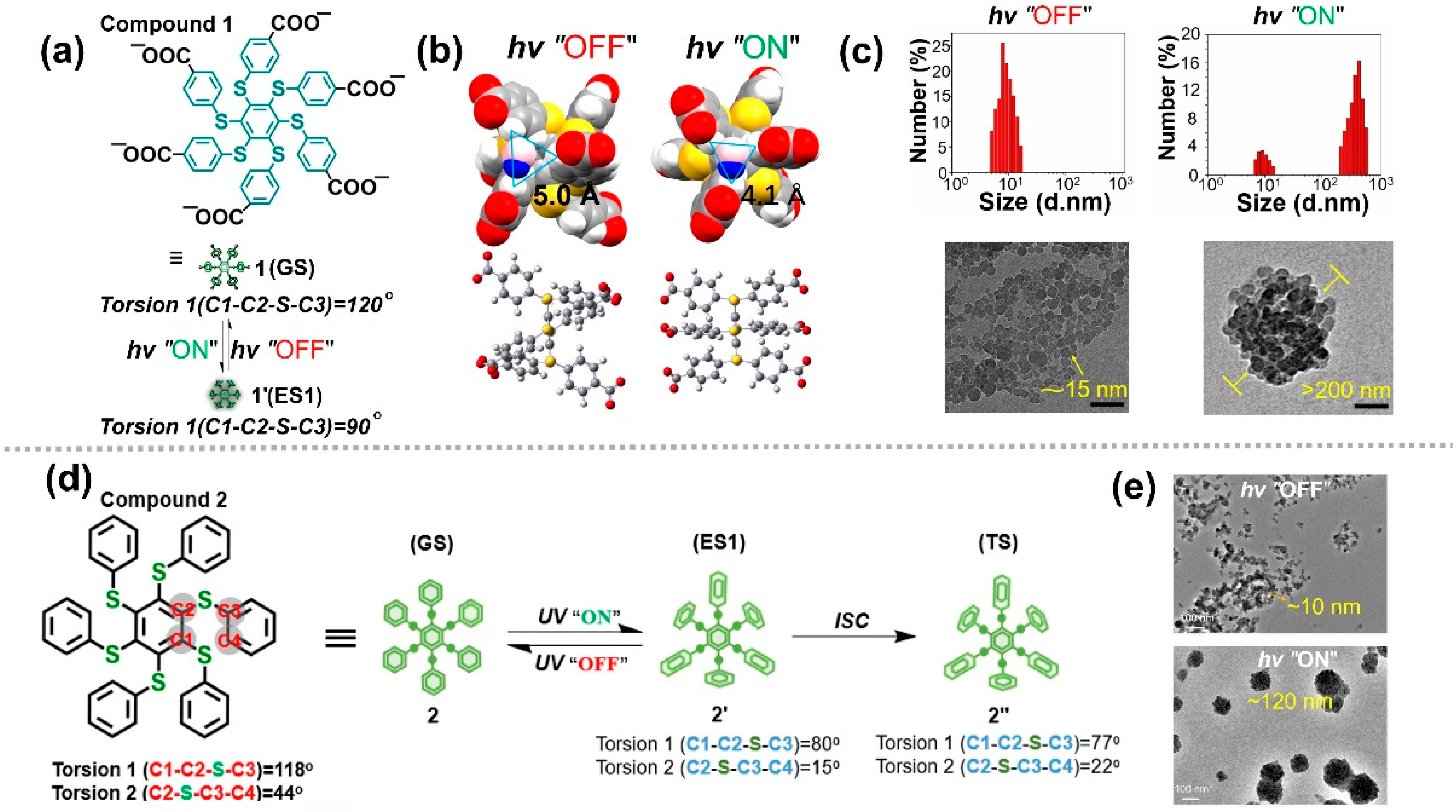
Figure 71. (a) Schematic illustration of the proposed conformational change upon photoexcitation of compound 1. (b) Equilibrium geometry of the GS and ES1 state of compound 1. (c) DLS results (top) and TEM images (bottom) of compound 1 in water before and after irradiation for 5 min [93][17]. (d) Calculated conformational change upon photoexcitation of compound 2. (e) TEM images of compound 2 in organic solvent before and after irradiation [94][18].
To further study the photo-controlled molecular aggregation caused by the change in excited state conformation in organic solvents, an oil-persulfurated arene molecule (compound 2, Figure 71d) was used in the research [94][18]. The theoretical calculation results show that there are obvious changes between ground and excited state conformations. Torsion 1 can change from 118° to 80°, whereas torsion 2 can change from 44° to 15°, proving that these molecules have significant spatial conformational conversion capabilities. The transmission electron microscopy images show that the particle size gradually changes from a few nanometers (10 nm) to hundreds of nanometers (120 nm), which is consistent with the dynamic light scattering results (Figure 71d). Therefore, this type of molecule is proven to have the ability of obvious spatial conformation transformation, which opens the possibility of photo-regulating molecular assemblies. By utilizing this feature, not only can the reversible aggregation of light-driven molecules be achieved, but also photo-controlled crystal growth and realignment can be achieved [95,96][19][20].
2.2. Photo-Controlled NP Aggregation
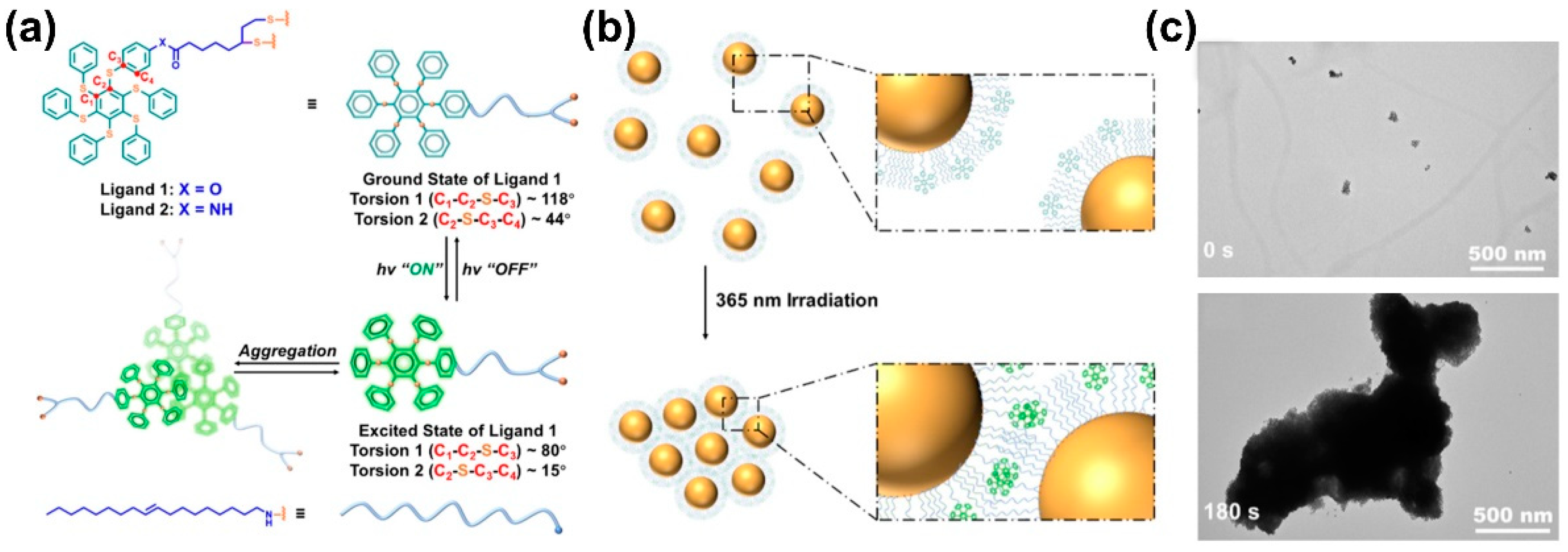
Inspired by the unique photoexcited, controlled aggregation properties of persulfurated benzenes, if such molecules are used to modify NPs, excited state-controlled self-assembly may be realized [97][21]. Zhu et al. prepared Au NPs with ligand 1 to modify the surface (Figure 82a). Through the theoretical calculation results, after the conformation change from the ground state to the excited state, torsion 1 (C1-C2-S-C3) can change from 118° to 80°, whereas torsion 2 (C2-S-C3-C4) can change from 44° to 15°. The excited state conformation promotes molecular aggregation of NPs as an assembly driving force. The initial Au NPs can disperse well in toluene solution and form particles with a diameter not greater than 10 nm, whereas these Au NPs particles can self-assemble into large size NP aggregates with a 1 µm diameter after photo irradiation for 3 min (Figure 82b,c). This study proves that molecular aggregation caused by the excited state conformational change can be used as a driving force to drive the self-assembly of NPs, further enriching the molecular assembly system controlled by photophysical processes.
References
- Yang, J.; Zhen, X.; Wang, B.; Gao, X.; Ren, Z.; Wang, J.; Xie, Y.; Li, J.; Peng, Q.; Pu, K.; et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat. Commun. 2018, 9, 840.
- Gu, L.; Shi, H.; Gu, M.; Ling, K.; Ma, H.; Cai, S.; Song, L.; Ma, C.; Li, H.; Xing, G.; et al. Dynamic Ultralong Organic Phosphorescence by Photoactivation. Angew. Chem. Int. Ed. Engl. 2018, 57, 8425–8431.
- Xu, S.; Chen, R.; Zheng, C.; Huang, W. Excited State Modulation for Organic Afterglow: Materials and Applications. Adv. Mater. 2016, 28, 9920–9940.
- Hu, H.; Cheng, X.; Ma, Z.; Sijbesma, R.P.; Ma, Z. Polymer Mechanochromism from Force-Tuned Excited-State Intramolecular Proton Transfer. J. Am. Chem. Soc. 2022, 144, 9971–9979.
- Chen, K.; Zhang, R.; Li, G.; Li, B.; Ma, Y.; Sun, M.; Wang, Z.; Tang, B.Z. Photo-induced crystallization with emission enhancement (PICEE). Mater. Horiz. 2020, 7, 3005–3010.
- Zhao, W.; Liu, Z.; Yu, J.; Lu, X.; Lam, J.W.Y.; Sun, J.; He, Z.; Ma, H.; Tang, B.Z. Turning On Solid-State Luminescence by Phototriggered Subtle Molecular Conformation Variations. Adv. Mater. 2021, 33, e2006844.
- Zhang, H.; Du, L.; Wang, L.; Liu, J.; Wan, Q.; Kwok, R.T.K.; Lam, J.W.Y.; Phillips, D.L.; Tang, B.Z. Visualization and Manipulation of Molecular Motion in the Solid State through Photoinduced Clusteroluminescence. J. Phys. Chem. Lett. 2019, 10, 7077–7085.
- Jia, X.; Yue, B.; Zhou, L.; Niu, X.; Wu, W.; Zhu, L. Fluorescence to multi-colored phosphorescence interconversion of a novel, asterisk-shaped luminogen via multiple external stimuli. Chem. Commun. 2020, 56, 4336–4339.
- Wang, J.; Zhang, M.; Han, S.; Zhu, L.; Jia, X. Multiple-stimuli-responsive multicolor luminescent self-healing hydrogel and application in information encryption and bioinspired camouflage. J. Mater. Chem. C 2022, 10, 15565–15572.
- Xu, J.; Feng, H.; Teng, H.; Chen, G.; Pan, S.; Chen, J.; Qian, Z. Reversible Switching between Phosphorescence and Fluorescence in a Unimolecular System Controlled by External Stimuli. Chem. Eur. J. 2018, 24, 12773–12778.
- Wu, H.; Chi, W.; Baryshnikov, G.; Wu, B.; Gong, Y.; Zheng, D.; Li, X.; Zhao, Y.; Liu, X.; Agren, H.; et al. Crystal Multi-Conformational Control Through Deformable Carbon-Sulfur Bond for Singlet-Triplet Emissive Tuning. Angew. Chem. Int. Ed. Engl. 2019, 58, 4328–4333.
- Fermi, A.; Bergamini, G.; Roy, M.; Gingras, M.; Ceroni, P. Turn-on phosphorescence by metal coordination to a multivalent terpyridine ligand: A new paradigm for luminescent sensors. J. Am. Chem. Soc. 2014, 136, 6395–6400.
- Wu, H.; Zhou, Y.; Yin, L.; Hang, C.; Li, X.; Agren, H.; Yi, T.; Zhang, Q.; Zhu, L. Helical Self-Assembly-Induced Singlet-Triplet Emissive Switching in a Mechanically Sensitive System. J. Am. Chem. Soc. 2017, 139, 785–791.
- Gu, J.; Yue, B.; Baryshnikov, G.V.; Li, Z.; Zhang, M.; Shen, S.; Agren, H.; Zhu, L. Visualizing Material Processing via Photoexcitation-Controlled Organic-Phase Aggregation-Induced Emission. Research 2021, 2021, 9862093.
- Yue, B.; Jia, X.; Baryshnikov, G.V.; Jin, X.; Feng, X.; Lu, Y.; Luo, M.; Zhang, M.; Shen, S.; Agren, H.; et al. Photoexcitation-Based Supramolecular Access to Full-Scale Phase-Diagram Structures through in situ Phase-Volume Ratio Phototuning. Angew. Chem. Int. Ed. Engl. 2022, 61, e202209777.
- Yue, B.; Feng, X.; Wang, C.; Zhang, M.; Lin, H.; Jia, X.; Zhu, L. In Situ Regulation of Microphase Separation-Recognized Circularly Polarized Luminescence via Photoexcitation-Induced Molecular Aggregation. ACS Nano 2022, 16, 16201–16210.
- Jia, X.; Shao, C.; Bai, X.; Zhou, Q.; Wu, B.; Wang, L.; Yue, B.; Zhu, H.; Zhu, L. Photoexcitation-controlled self-recoverable molecular aggregation for flicker phosphorescence. Proc. Natl. Acad. Sci. USA 2019, 116, 4816–4821.
- Weng, T.; Zou, Q.; Zhang, M.; Wu, B.; Baryshnikov, G.V.; Shen, S.; Chen, X.; Agren, H.; Jia, X.; Zhu, L. Enhancing the Operability of Photoexcitation-Controlled Aggregation-Induced Emissive Molecules in the Organic Phase. J. Phys. Chem. Lett. 2021, 12, 6182–6189.
- Shen, S.; Baryshnikov, G.; Yue, B.; Wu, B.; Li, X.; Zhang, M.; Ågren, H.; Zhu, L. Manipulating crystals through photoexcitation-induced molecular realignment. J. Mater. Chem. C 2021, 9, 11707–11714.
- Shen, S.; Baryshnikov, G.V.; Xie, Q.; Wu, B.; Lv, M.; Sun, H.; Li, Z.; Agren, H.; Chen, J.; Zhu, L. Making multi-twisted luminophores produce persistent room-temperature phosphorescence. Chem. Sci. 2023, 14, 970–978.
- Xu, X.; Zhang, M.; Li, Z.; Ye, D.; Gou, L.; Zou, Q.; Zhu, L. Highly efficient light-induced self-assembly of gold nanoparticles promoted by photoexcitation-induced aggregatable ligands. Chem. Commun. 2023, 59, 418–421.
More