Breast cancer (BC) is a lethal malignancy with high morbidity and mortality but lacks effective treatments thus far. Histone deacetylases 2 (HDAC2) inhibitor (HDAC2i) has been proven to exhibit an anti-cancer effect, can act as a sensitizer for immune checkpoint inhibitors (ICIs)ICIs therapy. Simultaneously, dietary intervention, as a crucial supportive therapy, has been reported to provide ingredients containing HDAC2 inhibitory activity. Thus, the novel integration of dietary intervention with ICIs therapy may offer promising possibilities for improving treatment outcomes.
- dietotherapy
- breast carcinoma
- HDAC2 suppression
- immune checkpoint inhibitor
1. Introduction
2. Dietary HDAC2i in Breast Cancer
2.1. HDAC2: A Potential Index of Aggressiveness and a Therapeutic Target against BC
HDAC2 is an enzyme involved in the tightening of the chromatin structure and suppression of gene transcription. Evidence has indicated that HDAC2 is overexpressed in breast cancer cells compared to normal breast tissue. Higher levels of HDAC2 have also been associated with more aggressive tumor characteristics, such as increased cell proliferation, invasion, and metastasis [12][13][14][15][12,13,14,15]. For instance, a clinical study that included 226 BC patients reported that expression of HDAC2 protein is significantly higher in breast cancer than in benign tumors and indicates that HDAC2 may be involved in invasion, metastasis, anthracyclines therapy resistance, and poor prognosis of sporadic breast cancer.2.2. HDAC2 Inhibition for Treating Breast Cancer
HDAC2 has been identified as a crucial regulator of epigenetic control in BC and HDAC2 suppression has been further proved to be an effective approach to treating BC by numerous studies [21][22][23][24,25,26]. For instance, HDAC2 inhibition has been observed to inhibit cellular proliferation in a p53-dependent manner in BC cells [24][27]. MiR-155 can also decrease the expression of erythroblastic oncogene B by targeting HDAC2 [25][28]. Moreover, evidence has indicated that PELP1 (proline, glutamate, and leucine-rich protein 1) can bind to miR-200a and miR-141 promoter sequences and modulate the expression of these miRNAs by recruiting HDAC2; therefore, regulating tumorigenic and metastatic potential of BC cells [26][29]. Thus, a molecular network involving HDAC2 has been considered to serve as a target for developing anti-cancer drugs [26][29]. Inhibition of HDAC2 has also been found to exert a synergistic effect in BC treatment. For instance, evidence indicates that depleting HDAC2 can sensitize breast cancer cells to apoptosis induced by epirubicin. This finding highlighted the potential of HDAC2 as a therapeutic target and a biomarker in treating breast cancer [27][30]. Moreover, the modulation of estrogen receptor (ER) signaling is a promising therapeutic approach in ER-expressing breast cancers, and the progesterone receptor (PR) also plays a critical role in this process [28][31].2.3. HDAC2 Inhibition Enhances the Therapeutic Effect of ICIs in BC Treatment
The emergence of immune checkpoint inhibitors (ICI) has revolutionized the treatment of breast cancer [29][30][31][32][33][34][35][36][33,34,35,36,37,38,39,40]. Adjuvant or neoadjuvant immune checkpoint blockades are used for metastatic breast cancer [30][34]. Despite ICI therapy being promising, breast cancer cells often find ways to evade the host’s immune system, necessitating combination therapies to overcome these limitations. HDAC inhibitors (HDACi) have demonstrated potent immunomodulatory activity, making them a rational choice for cancer immunotherapies.
2.3.1. HDAC2 Regulates PD-L1 Nuclear Translocation
As a ligand of PD-1, high PD-L1 levels indicate tumor progression and are associated with poor prognosis in immunotherapy-treated human cancer [31][35]. PD-L1 nuclear translocation has been identified as a key mechanism underlying the immune evasion of BC cells, hindering PD-1 inhibitors [32][36]. Both cytoplasmic and nuclear PD-L1 can exert immunosuppressive functions on BC cells [33][37]. Nuclear PD-L1 has been linked to various cellular processes, for example, increasing the anti-apoptotic capacity of tumor cells, promoting mTOR activity, and upregulating glycolytic metabolism [32][36]. A study has shown that the level of nuclear PD-L1 expression was positively correlated with immune response-related transcription factors, such as STAT3, RelA (p65), and c-Jun [19]. Nuclear PD-L1 interacts with transcription factors, such as RelA and the IFN regulatory factor (IRF), influencing antitumor immunity. Inhibition of nuclear PD-L1 expression led to downregulation of genes involved in evading immune surveillance, such as PDCD1LG2 (encoding PD-L2), VSIR (encoding VISTA), and CD276 (encoding B7-H3), which enhance cytotoxic T-lymphocyte depletion and promote tumor aggressiveness, distant metastasis, and resistance to PD-L1/PD-1 blockade therapy [18][19][34][18,19,38] (Figure 12).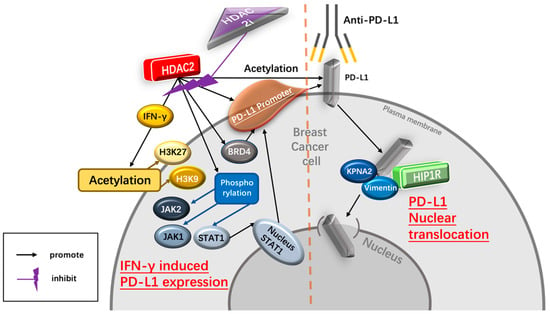
2.3.2. HDAC2 Regulates IFN-γ- Induced PD-L1 Expression
IFN-γ upregulates the expression of PD-L1 Interferon-γ (IFN-γ) is a crucial cytokine in both innate and adaptive immunity and should be considered as an important driving force for PD-L1 expression in tumor microenvironment. It is able to induce PD-L1 expression on BC cells and increase the apoptosis of antigen-specific T cells, such a process is referred to as “adaptive resistance” [37][43]. Depleting IFN-γ receptor 1 has been reported to decrease the expression of PD-L1 expression in BC cells, increase the amount of tumor-infiltrating CD8+ lymphocytes or CTLs, and to inhibit the development of BC cells [38][44]. HDAC2i affects IFN-γ induced PD-L1 HDAC2 can promote PD-L1 induced via IFN-γ stimulation in BC cells [18][19][18,19]. Specifically, IFN-γ can induce gene transcription involving STAT1 binding to the gamma interferon activation site (GAS), recruiting HAT and HDAC for chromatin remodeling [39][45]. Following IFN-γ stimulation, BRD4 is rapidly recruited to the PD-L1 locus, accompanied by increased H3K27ac and RNA Polymerase II (RNA Pol II) occupancy in cancer cells [39][45]. A ChIP-qPCR assay confirmed enhanced HDAC2 binding to the PD-L1 promoter after IFN-γ treatment. HDAC2 knockout led to reduced STAT1 occupancy and bromodomain-containing 4 (BRD4) recruitment to the PD-L1 promoter, attenuated H3K27ac and H3K9ac (markers of active transcription in the PD-L1 promoter) upregulation induced by IFN-γ, highlighting HDAC2’s role in activating PD-L1 expression through IFN-γ induced signaling pathways [18].2.4. Dietary Intervention Is Important for Breast Cancer Patients Receiving Anti-Cancer Immunotherapy
A healthy diet rich in vitamins, minerals, antioxidants, and phytochemicals, provides essential ingredients for building and strengthening a healthy immune system. Thus, the American Cancer Society (ACS) releases the Nutrition and Physical Activity Guideline for Cancer Survivors, which enhances immune function by supporting essential nutrients [40][41][42][43][47,48,49,50], helping to optimize the effectiveness of immunotherapy and improve treatment outcomes. Moreover, anti-cancer immunotherapy also possesses toxicities and can lead to various adverse effects on breast cancer patients, such as fatigue, nausea, loss of appetite, and gastrointestinal issues [44][51]. However, proper dietary interventions have been found to help alleviate these side effects by providing proper nutrition, promoting hydration, and supporting gastrointestinal health [45][46][47][52,53,54].2.5. Dietary HDAC2i
Compared to commonly used anti-cancer therapies, dietary interventions are safer and more cost-effective [48][49][65,66]. Even if dietary interventions cannot be considered a replacement for conventional cancer treatments, their role in improving the outcomes of cancer treatment also cannot be ignored [50][51][67,68] (Figure 23).