You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Jitendra Kumar Sinha.
Epilepsy is a complex neurological disorder affecting millions worldwide, with a substantial number of patients facing drug-resistant epilepsy. Conventional antiseizure medications (ASMs) have been the cornerstone of epilepsy management, providing significant relief to a large number of patients In the context of epilepsy, gene therapy holds the potential to address the underlying genetic abnormalities that give rise to seizure disorders. By targeting specific genes associated with epilepsy, gene therapy aims to restore normal cellular function and inhibit seizure generation, providing a promising avenue for the development of novel and more targeted epilepsy treatments.
- epilepsy
- gene therapies
1. Introduction
Epilepsy, a chronic neurological disorder characterized by recurrent seizures, affects millions of people worldwide [1,2,3][1][2][3]. Seizures result from abnormal electrical activity in the brain, leading to various physical and cognitive manifestations. While traditional antiseizure medications (ASMs) have been the cornerstone of epilepsy management for decades, a significant proportion of patients continue to experience seizures despite treatment [3]. This has spurred the exploration of innovative therapies and emerging trends in epilepsy management to address the unmet medical needs of individuals living with drug-resistant epilepsy [2]. The management of epilepsy has come a long way since its earliest descriptions in ancient texts, but challenges persist [4]. Traditional ASMs can be associated with adverse effects, including cognitive impairments, mood disturbances, and systemic toxicity [5]. Additionally, certain epilepsy syndromes may be particularly refractory to conventional treatments, necessitating alternative approaches to achieve better outcomes [6].
2. Traditional Approaches to Epilepsy Management
For decades, conventional ASMs have been the cornerstone of epilepsy management, providing significant relief to a large number of patients [8,9][7][8]. ASMs work by modulating the excitability of neurons, inhibiting the abnormal electrical activity that triggers seizures [10][9]. The advent of these medications has revolutionized epilepsy treatment and has been instrumental in achieving seizure control and improving the quality of life for many individuals with epilepsy [11][10]. Various classes of ASMs are available, each targeting specific mechanisms involved in seizure generation and propagation [12][11]. Common ASMs include phenytoin, carbamazepine, valproate, lamotrigine, and levetiracetam [13,14][12][13]. These drugs are typically prescribed based on the patient’s seizure type, epilepsy syndrome, age, and overall health. Despite their widespread use and effectiveness in a significant proportion of patients, ASMs have limitations that can hinder optimal epilepsy management. First, not all patients respond favorably to traditional ASMs, leading to drug-resistant epilepsy. Estimates suggest that approximately one-third of people with epilepsy continue to experience seizures despite adequate trials of two or more ASMs [15][14]. This phenomenon poses a significant clinical challenge and underscores the need for alternative therapeutic approaches to address drug-resistant epilepsy.2.1. Challenges in Achieving Seizure Control with Traditional Therapies
Drug-resistant epilepsy represents a major clinical hurdle in epilepsy management [16][15]. Patients who are refractory to traditional ASMs face recurrent seizures that can severely impact their daily lives, disrupt social interactions, and limit educational and employment opportunities [17,18,19][16][17][18]. The unpredictable nature of seizures can lead to anxiety, depression, and a reduced overall quality of life. The reasons behind treatment resistance in epilepsy are complex and multifactorial. One significant challenge is the inherent variability in epilepsy. The condition is heterogeneous, and the underlying causes and mechanisms can differ greatly from one patient to another. As a result, ASMs that are effective for some individuals may not work as well for others due to differences in the brain’s structure and function [20,21][19][20]. The diversity in epilepsy subtypes, seizure types, and responses to treatment makes it challenging to achieve uniform seizure control with traditional therapies [22][21]. Pharmacokinetic variability is another factor contributing to treatment resistance [23][22]. The way ASMs are metabolized and distributed in the body can vary among individuals, affecting drug levels and therapeutic efficacy. Drug interactions and genetic factors can also influence AED metabolism, leading to differences in drug response and treatment outcomes [24,25][23][24]. This variability in drug levels can result in suboptimal seizure control and contribute to treatment resistance. Moreover, the mechanisms of action of ASMs may not address all aspects of seizure generation and propagation. While these medications primarily target ion channels and neurotransmitter receptors, certain epilepsy syndromes may involve complex networks of neurons, making them less responsive to the effects of traditional ASMs [26][25]. As a result, treatment with ASMs alone may not be sufficient to achieve complete seizure control in some cases [27,28][26][27]. Compliance issues also play a significant role in treatment resistance. Adherence to prescribed AED regimens is crucial for successful seizure management [29,30][28][29]. However, poor medication compliance can reduce the effectiveness of treatment and contribute to treatment resistance [29,30][28][29]. Factors such as forgetfulness, medication side effects, and the inconvenience of multiple daily doses can hinder patients’ consistent adherence to their treatment plans. Tolerance and adaptation are additional challenges in epilepsy management [31][30]. Over time, some individuals may develop tolerance to the effects of ASMs, leading to decreased seizure control. The brain’s adaptability and compensatory mechanisms may reduce the long-term efficacy of certain medications, necessitating the need for alternative therapeutic approaches [32][31]. Furthermore, ASMs may be associated with side effects that impact treatment adherence and tolerability. Some patients may experience significant adverse effects such as dizziness, drowsiness, cognitive impairment, and mood disturbances [33][32]. For some individuals, these side effects may outweigh the benefits of seizure reduction, leading to treatment discontinuation or non-compliance. Addressing the challenges of drug-resistant epilepsy requires a comprehensive and individualized approach. As researchers delve into the world of emerging therapies, it is important to recognize that traditional ASMs continue to be vital in managing epilepsy for many patients [34][33]. However, the limitations of these therapies underscore the need for innovative and personalized treatments. By understanding the complexities of treatment resistance and identifying novel targets, such as specific genes or neural circuits, researchers can develop more effective therapies to improve seizure control and enhance the quality of life for individuals living with epilepsy. As researchers move forward, collaboration between researchers, clinicians, and patients will play a pivotal role in advancing the field of epilepsy management, driving us closer to the day when drug-resistant epilepsy becomes a challenge of the past [35][34]. The pursuit of emerging trends and innovative therapies, along with a deeper understanding of the underlying mechanisms of epilepsy, offers hope for improved therapeutic options and a brighter future for people living with epilepsy. Through continued research, dedication, and unwavering commitment, researchers can transform the lives of those affected by epilepsy and pave the way for more effective and personalized treatments.2.2. The Need for Novel Treatment Approaches to Address Drug-Resistant Epilepsy
The persistence of drug-resistant epilepsy highlights the critical necessity for novel and innovative treatment approaches [22,36,37][21][35][36]. Research and clinical efforts have intensified in recent years to develop therapies that target specific epilepsy subtypes, identify novel drug targets, and explore non-pharmacological interventions. The emergence of new technologies and a deeper understanding of the underlying mechanisms of epilepsy have paved the way for innovative therapeutic strategies [22][21]. Neurostimulation devices, such as RNS, VNS, and DBS, offer potential alternatives for patients who are unresponsive to traditional ASMs (Figure 1) [37][36]. Additionally, advancements in precision medicine and personalized approaches hold promise for tailoring treatments to individual patients based on their unique genetic and molecular profiles [38,39][37][38] Gene therapies are also being explored as potential treatments for certain genetic epilepsy syndromes [40][39].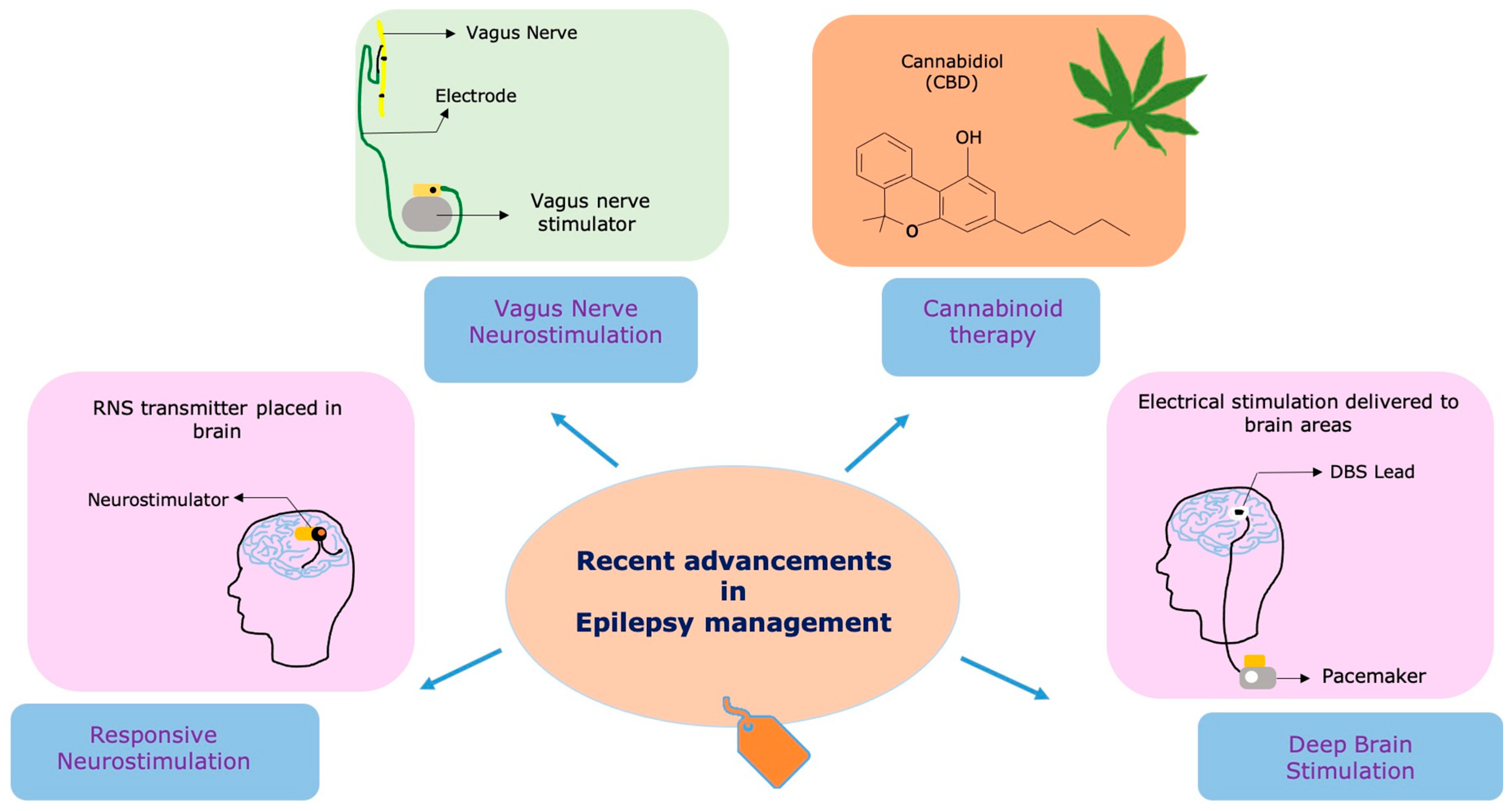
Figure 1. Recent technologies and therapies used in the management of epilepsy. The figure illustrates cutting-edge treatments and innovative therapies utilized in epilepsy management. Responsive neurostimulation (RNS)—An implantable neurostimulator device that detects and responds to abnormal brain activity, providing on-demand electrical stimulation to reduce seizure frequency. Vagus nerve stimulation (VNS)—A device that stimulates the vagus nerve through electrical impulses, helping to modulate brain activity and decrease seizure occurrences. Deep brain stimulation (DBS)—A neuromodulation technique that involves implanting electrodes in specific brain regions to deliver electrical stimulation and regulate neural activity for seizure control. Cannabidiol (CBD)—A natural compound derived from the cannabis plant, known for its potential anticonvulsant effects and often used as an adjunct therapy for certain epilepsy syndromes.
3. Gene Therapies for Epilepsy
Gene therapy is an innovative approach aimed at treating diseases by modifying or manipulating the genetic material of cells [190,191][42][43]. The fundamental principle of gene therapy is to correct or replace faulty genes that contribute to the development or progression of a particular condition. In the context of epilepsy, gene therapy holds the potential to address the underlying genetic abnormalities that give rise to seizure disorders [192][44]. By targeting specific genes associated with epilepsy, gene therapy aims to restore normal cellular function and inhibit seizure generation, providing a promising avenue for the development of novel and more targeted epilepsy treatments [40,186,193][39][45][46].
Recent advancements in gene editing technologies have revolutionized the field of gene therapy, enabling more precise and efficient targeting of specific genes. One of the most revolutionary gene editing tools is CRISPR-Cas9, which allows scientists to edit DNA sequences with remarkable accuracy [194][47]. With CRISPR-Cas9, researchers can modify or delete epilepsy-related genes and investigate their impact on seizure susceptibility [194][47]. For epilepsy, gene editing techniques are being utilized to explore the role of various genes implicated in the disorder. By targeting genes associated with ion channels, neurotransmitter receptors, or cellular signaling pathways, researchers can investigate how alterations in these genes contribute to epileptogenesis [195,196][48][49]. Additionally, gene editing tools are being used to correct disease-causing mutations in patient-derived cells or animal models, potentially paving the way for personalized gene therapies tailored to specific genetic defects [197,198,199,200][50][51][52][53].
Safety and Efficacy of Gene Therapies
While gene therapy for epilepsy is still in the early stages of development, preclinical studies in animal models have shown promising results [184,201,202][54][55][56]. Animal models with specific epilepsy-related genetic mutations have been treated using gene therapy approaches, resulting in reduced seizure frequency and improved seizure control [202,203,204,205][56][57][58][59]. The preclinical studies have provided valuable insights into the potential therapeutic benefits of gene therapies and have identified potential target genes for further investigation [205][59]. In terms of clinical studies, several gene therapy trials for epilepsy are currently underway or in the planning stages. These trials aim to evaluate the safety and efficacy of gene therapies in human patients with specific genetic forms of epilepsy. One notable example is the development of adeno-associated virus (AAV) vectors as a delivery system for gene therapies [206][60]. AAV vectors have shown promise as a means to deliver therapeutic genes to specific brain regions in a controlled and targeted manner [206,207][60][61]. It is important to note that gene therapy approaches for epilepsy face unique challenges. The complexity of the brain and the diversity of genetic factors contributing to epilepsy necessitate rigorous evaluation of potential risks and benefits. Delivery methods, such as viral vectors, must be carefully engineered to ensure accurate and efficient gene transfer while minimizing immune responses and other adverse effects [207,208][61][62].
Long-term safety and potential off-target effects of gene editing in the brain remain areas of active investigation and consideration [209][63]. Ethical considerations, such as ensuring informed consent and addressing concerns about permanent genetic modifications, are crucial in developing responsible gene therapies for epilepsy [191][43]. Nevertheless, gene therapy represents a promising and innovative frontier in epilepsy treatment [210][64]. By targeting specific genes associated with epileptogenesis, gene therapies hold the potential to provide more precise and personalized treatments for individuals with genetic forms of epilepsy [211][65]. While challenges remain, gene therapies have the potential to revolutionize epilepsy treatment and improve the lives of individuals living with this challenging neurological disorder.
Emerging advancements in gene therapy hold significant promise for revolutionizing the landscape of epilepsy treatment. Gene therapy offers a groundbreaking approach to address the genetic anomalies contributing to seizure disorders by manipulating cellular genetic material. Through gene editing technologies such as CRISPR-Cas9, researchers can now target specific epilepsy-associated genes with remarkable accuracy, shedding light on the intricate molecular mechanisms underlying epileptogenesis. Recent preclinical studies in animal models demonstrate promising results, with gene therapy interventions effectively reducing seizure frequency and enhancing seizure control. Clinical trials focusing on human patients with specific genetic forms of epilepsy are underway, evaluating the safety and efficacy of gene therapies. While challenges in delivering genes to the brain and ensuring long-term safety remain, gene therapy’s potential to provide precise, personalized treatments for genetic forms of epilepsy is groundbreaking. Ethical considerations and rigorous evaluation are imperative, but the prospects of gene therapies offer hope for revolutionizing epilepsy treatment and improving the quality of life for those impacted by this complex neurological disorder.
References
- Jacoby, A.; Snape, D.; Baker, G.A. Epilepsy and Social Identity: The Stigma of a Chronic Neurological Disorder. Lancet Neurol. 2005, 4, 171–178.
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; de Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Prim. 2018, 4, 18024.
- Ghosh, S.; Sinha, J.K.; Khan, T.; Devaraju, K.S.; Singh, P.; Vaibhav, K.; Gaur, P. Pharmacological and Therapeutic Approaches in the Treatment of Epilepsy. Biomedicines 2021, 9, 470.
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia 2014, 55, 475–482.
- Reddy, D.S. Therapeutic and Clinical Foundations of Cannabidiol Therapy for Difficult-to-Treat Seizures in Children and Adults with Refractory Epilepsies. Exp. Neurol. 2023, 359, 114237.
- Fattorusso, A.; Matricardi, S.; Mencaroni, E.; Dell’Isola, G.B.; Di Cara, G.; Striano, P.; Verrotti, A. The Pharmacoresistant Epilepsy: An Overview on Existant and New Emerging Therapies. Front. Neurol. 2021, 12, 674483.
- Anwar, H.; Khan, Q.U.; Nadeem, N.; Pervaiz, I.; Ali, M.; Cheema, F.F. Epileptic Seizures. Discoveries 2020, 8, e110.
- Mishra, P.; Sinha, J.K.; Rajput, S.K. Efficacy of Cicuta Virosa Medicinal Preparations against Pentylenetetrazole-Induced Seizures. Epilepsy Behav. 2021, 115, 107653.
- Rogawski, M.A.; Löscher, W.; Rho, J.M. Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harb. Perspect. Med. 2016, 6, a022780.
- Baker, G.A.; Jacoby, A.; Buck, D.; Stalgis, C.; Monnet, D. Quality of Life of People with Epilepsy: A European Study. Epilepsia 1997, 38, 353–362.
- Baftiu, A.; Lima, M.H.; Svendsen, K.; Larsson, P.G.; Johannessen, S.I.; Landmark, C.J. Safety Aspects of Antiepileptic Drugs-a Population-Based Study of Adverse Effects Relative to Changes in Utilisation. Eur. J. Clin. Pharmacol. 2019, 75, 1153–1160.
- Asconapé, J.J. Some Common Issues in the Use of Antiepileptic Drugs. Semin Neurol. 2002, 22, 27–39.
- Walia, K.S.; Khan, E.A.; Ko, D.H.; Raza, S.S.; Khan, Y.N. Side Effects of Antiepileptics—A Review. Pain Pract. 2004, 4, 194–203.
- Stevelink, R.; Koeleman, B.P.C.; Sander, J.W.; Jansen, F.E.; Braun, K.P.J. Refractory Juvenile Myoclonic Epilepsy: A Meta-Analysis of Prevalence and Risk Factors. Eur. J. Neurol. 2019, 26, 856–864.
- Schmidt, D.; Löscher, W. Drug Resistance in Epilepsy: Putative Neurobiologic and Clinical Mechanisms. Epilepsia 2005, 46, 858–877.
- Nickels, K.C.; Zaccariello, M.J.; Hamiwka, L.D.; Wirrell, E.C. Cognitive and Neurodevelopmental Comorbidities in Paediatric Epilepsy. Nat. Rev. Neurol. 2016, 12, 465–476.
- Okiah, L.; Olowo, S.; Iramiot, S.J.; Nekaka, R.; Ssenyonga, L.V.N. Lived Experiences of Caregivers of Persons with Epilepsy Attending an Epilepsy Clinic at a Tertiary Hospital, Eastern Uganda: A Phenomenological Approach. PLoS ONE 2023, 18, e0274373.
- Camfield, P.R.; Camfield, C.S. What Happens to Children with Epilepsy When They Become Adults? Some Facts and Opinions. Pediatr. Neurol. 2014, 51, 17–23.
- Laxer, K.D.; Trinka, E.; Hirsch, L.J.; Cendes, F.; Langfitt, J.; Delanty, N.; Resnick, T.; Benbadis, S.R. The Consequences of Refractory Epilepsy and Its Treatment. Epilepsy Behav. 2014, 37, 59–70.
- Duncan, J.S.; Sander, J.W.; Sisodiya, S.M.; Walker, M.C. Adult Epilepsy. Lancet 2006, 367, 1087–1100.
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638.
- Johannessen Landmark, C.; Johannessen, S.I.; Tomson, T. Host Factors Affecting Antiepileptic Drug Delivery-Pharmacokinetic Variability. Adv. Drug Deliv. Rev. 2012, 64, 896–910.
- Urzì Brancati, V.; Pinto Vraca, T.; Minutoli, L.; Pallio, G. Polymorphisms Affecting the Response to Novel Antiepileptic Drugs. Int. J. Mol. Sci. 2023, 24, 2535.
- Patsalos, P.N.; Perucca, E. Clinically Important Drug Interactions in Epilepsy: General Features and Interactions between Antiepileptic Drugs. Lancet Neurol. 2003, 2, 347–356.
- Chen, T.-S.; Lai, M.-C.; Huang, H.-Y.I.; Wu, S.-N.; Huang, C.-W. Immunity, Ion Channels and Epilepsy. Int. J. Mol. Sci. 2022, 23, 6446.
- Rogawski, M.A.; Löscher, W. The Neurobiology of Antiepileptic Drugs. Nat. Rev. Neurosci. 2004, 5, 553–564.
- Kobayashi, K.; Endoh, F.; Ohmori, I.; Akiyama, T. Action of Antiepileptic Drugs on Neurons. Brain Dev. 2020, 42, 2–5.
- Liu, J.; Xu, R.; Liu, Z.; You, Y.; Meng, F. Factors Influencing Medication Adherence after Epilepsy Surgery. Epileptic Disord. 2015, 17, 47–51.
- Faught, E. Adherence to Antiepilepsy Drug Therapy. Epilepsy Behav. 2012, 25, 297–302.
- Wahab, A. Difficulties in Treatment and Management of Epilepsy and Challenges in New Drug Development. Pharmaceuticals 2010, 3, 2090–2110.
- Löscher, W.; Schmidt, D. Experimental and Clinical Evidence for Loss of Effect (Tolerance) during Prolonged Treatment with Antiepileptic Drugs. Epilepsia 2006, 47, 1253–1284.
- Kennedy, G.M.; Lhatoo, S.D. CNS Adverse Events Associated with Antiepileptic Drugs. CNS Drugs 2008, 22, 739–760.
- Elger, C.E.; Schmidt, D. Modern Management of Epilepsy: A Practical Approach. Epilepsy Behav. 2008, 12, 501–539.
- Shih, P.; Francis-Auton, E.; Nikpour, A.; Herkes, G.; Bleasel, A.; Rapport, F. Enhancing Quality of Life among Epilepsy Surgery Patients: Interlinking Multiple Social and Relational Determinants. Epilepsy Behav. 2020, 102, 106721.
- Sinha, N.; Johnson, G.W.; Davis, K.A.; Englot, D.J. Integrating Network Neuroscience Into Epilepsy Care: Progress, Barriers, and Next Steps. Epilepsy Curr. 2022, 22, 272–278.
- Singhal, N.S.; Numis, A.L.; Lee, M.B.; Chang, E.F.; Sullivan, J.E.; Auguste, K.I.; Rao, V.R. Responsive Neurostimulation for Treatment of Pediatric Drug-Resistant Epilepsy. Epilepsy Behav. Case Rep. 2018, 10, 21–24.
- Li, Y.; Zhang, S.; Snyder, M.P.; Meador, K.J. Precision Medicine in Women with Epilepsy: The Challenge, Systematic Review, and Future Direction. Epilepsy Behav. 2021, 118, 107928.
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370.
- Perucca, E.; Perucca, P.; White, H.S.; Wirrell, E.C. Drug Resistance in Epilepsy. Lancet Neurol. 2023, 22, 723–734.
- Madireddy, S.; Madireddy, S. Therapeutic Strategies to Ameliorate Neuronal Damage in Epilepsy by Regulating Oxidative Stress, Mitochondrial Dysfunction, and Neuroinflammation. Brain Sci. 2023, 13, 784.
- Menon, S.; Sander, J.W. Effects of the COVID-19 Pandemic on Medication Adherence: In the Case of Antiseizure Medications, A Scoping Review. Seizure 2021, 93, 81–87.
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a Gene-Editing Approach to Restore Vision Loss in Leber Congenital Amaurosis Type 10. Nat. Med. 2019, 25, 229–233.
- Ghosh, S.; Ghosh, S.; Raghunath, M.; Bhaskar, R.; Sinha, J.K. Balancing Potential Benefits and Ethical Considerations of Gene Editing. Lancet 2023, 401, 2109–2110.
- Kullmann, D.M.; Schorge, S.; Walker, M.C.; Wykes, R.C. Gene Therapy in Epilepsy-Is It Time for Clinical Trials? Nat. Rev. Neurol. 2014, 10, 300–304.
- Stafstrom, C.E.; Carmant, L. Seizures and Epilepsy: An Overview for Neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426.
- Goodspeed, K.; Bailey, R.M.; Prasad, S.; Sadhu, C.; Cardenas, J.A.; Holmay, M.; Bilder, D.A.; Minassian, B.A. Gene Therapy: Novel Approaches to Targeting Monogenic Epilepsies. Front. Neurol. 2022, 13, 805007.
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-Fidelity CRISPR-Cas9 Nucleases with No Detectable Genome-Wide off-Target Effects. Nature 2016, 529, 490–495.
- Goldberg, E.M.; Coulter, D.A. Mechanisms of Epileptogenesis: A Convergence on Neural Circuit Dysfunction. Nat. Rev. Neurosci. 2013, 14, 337–349.
- Rakhade, S.N.; Jensen, F.E. Epileptogenesis in the Immature Brain: Emerging Mechanisms. Nat. Rev. Neurol. 2009, 5, 380–391.
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Signal Transduct. Target. Ther. 2020, 5, 1.
- Cai, L.; Fisher, A.L.; Huang, H.; Xie, Z. CRISPR-Mediated Genome Editing and Human Diseases. Genes Dis. 2016, 3, 244–251.
- Dhuriya, Y.K.; Naik, A.A. CRISPR: A Tool with Potential for Genomic Reprogramming in Neurological Disorders. Mol. Biol. Rep. 2023, 50, 1845–1856.
- Jensen, T.L.; Gøtzsche, C.R.; Woldbye, D.P.D. Current and Future Prospects for Gene Therapy for Rare Genetic Diseases Affecting the Brain and Spinal Cord. Front. Mol. Neurosci. 2021, 14, 695937.
- Hartman, A.L.; Gasior, M.; Vining, E.P.G.; Rogawski, M.A. The Neuropharmacology of the Ketogenic Diet. Pediatr. Neurol. 2007, 36, 281–292.
- Riban, V.; Fitzsimons, H.L.; During, M.J. Gene Therapy in Epilepsy. Epilepsia 2009, 50, 24–32.
- Simonato, M.; Bennett, J.; Boulis, N.M.; Castro, M.G.; Fink, D.J.; Goins, W.F.; Gray, S.J.; Lowenstein, P.R.; Vandenberghe, L.H.; Wilson, T.J.; et al. Progress in Gene Therapy for Neurological Disorders. Nat. Rev. Neurol. 2013, 9, 277–291.
- Morey, N.; Przybyla, M.; van der Hoven, J.; Ke, Y.D.; Delerue, F.; van Eersel, J.; Ittner, L.M. Treatment of Epilepsy Using a Targeted P38γ Kinase Gene Therapy. Sci. Adv. 2022, 8, eadd2577.
- Nabbout, R.; Kuchenbuch, M. Impact of Predictive, Preventive and Precision Medicine Strategies in Epilepsy. Nat. Rev. Neurol. 2020, 16, 674–688.
- Jacobs, M.P.; Leblanc, G.G.; Brooks-Kayal, A.; Jensen, F.E.; Lowenstein, D.H.; Noebels, J.L.; Spencer, D.D.; Swann, J.W. Curing Epilepsy: Progress and Future Directions. Epilepsy Behav. 2009, 14, 438–445.
- Weinberg, M.S.; Samulski, R.J.; McCown, T.J. Adeno-Associated Virus (AAV) Gene Therapy for Neurological Disease. Neuropharmacology 2013, 69, 82–88.
- Finer, M.; Glorioso, J. A Brief Account of Viral Vectors and Their Promise for Gene Therapy. Gene Ther. 2017, 24, 1–2.
- Kang, L.; Jin, S.; Wang, J.; Lv, Z.; Xin, C.; Tan, C.; Zhao, M.; Wang, L.; Liu, J. AAV Vectors Applied to the Treatment of CNS Disorders: Clinical Status and Challenges. J. Control. Release 2023, 355, 458–473.
- Morris, G.; Schorge, S. Gene Therapy for Neurological Disease: State of the Art and Opportunities for Next-Generation Approaches. Neuroscience 2022, 490, 309–314.
- Cattaneo, S.; Verlengia, G.; Marino, P.; Simonato, M.; Bettegazzi, B. NPY and Gene Therapy for Epilepsy: How, When,... and Y. Front. Mol. Neurosci. 2020, 13, 608001.
- EpiPM Consortium A Roadmap for Precision Medicine in the Epilepsies. Lancet Neurol. 2015, 14, 1219–1228.
More
