Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Aradhna Kumari.
Melatonin, a hormone known for its role in regulating sleep–wake cycles in mammals, has been found to have diverse functions in horticultural plants. In recent years, research has revealed the involvement of melatonin in various physiological processes in plants, like regulation of growth and development, stress tolerance, and antioxidant defense. Melatonin can augment seed germination, roots, shoot growth, and biomass accumulation in horticultural crops. It also performs a vital role in regulating vegetative and reproductive growth stages, floral transition, and leaf senescence. Melatonin improves stress tolerance in crops by regulating root architecture, nutrient uptake, and ion transport.
- melatonin
- biosynthesis
- physiological roles
- interaction with phytohormones
- horticultural crops
1. Melatonin Receptors in Plants
Melatonin receptors were first discovered in the mammalian brain in the early 1980s [38][1]. Later, melatonin receptors were identified in other tissues of mammals and other organisms such as birds, fish, amphibians, and invertebrates [38,45,46,47][1][2][3][4]. In plants, melatonin receptors were first discovered [48][5]. In Arabidopsis thaliana, specifically in a gene known asCAND2/PMTR1.This gene regulates stomatal closure via an H2O2 and Ca2+ signaling transduction cascade. More recently, studied CAND2 and proposed that the integrity of CAND2 as a melatonin receptor needs further analysis because their confocal microscopy analysis showed thatCAND2 protein is locali
Biosynthesis of Melatonin in Crops
Melatonin biosynthesis in plants occurs via the shikimate pathway, which is also related to the biosynthesis of several other important secondary metabolites in plants. The biosynthesis pathway converts the amino acid tryptophan into melatonin through several enzymatic reactions. The biosynthesis of melatonin starts with the conversion of tryptophan to serotonin by the enzyme tryptophan decarboxylase (TDC). After that, serotonin converted into N-acetyl serotonin (NAS) by the enzyme serotonin N-acetyltransferase (SNAT). Finally, NAS is converted into melatonin by the enzyme N-acetyl serotonin O-methyltransferase (ASMT) [31][6]. Abiotic stresses can influence the activity of these enzymes. For instance, research has shown that drought stress can enhance the expression of TDC, SNAT, and ASMT genes in rice, subsequently increasing melatonin biosynthesis [50][7]. In addition, plant hormones such as auxins and cytokinin have been shown to regulate melatonin biosynthesis in crops. Experiments have suggested that exogenous treatment of these phytohormones may increase plant melatonin biosynthesis [16,22][8][9].
Recent research has also uncovered the roles of other enzymes, such as caffeic acid O-methyltransferase (COMT) and acetyl serotonin deacetylase (ASDD), in the melatonin biosynthesis pathway. COMT is concerned with synthesizing the intermediate compound 5-methoxy tryptamine (5-MT), which is a precursor of NAS. At the same time, ASDD is involved in the deacetylation of NAS to form serotonin [1][10]. The melatonin biosynthesis pathway in crops is complex and regulated by various factors, including environmental stresses, plant hormones, and genetic factors. Further experiments are necessary to fully understand the regulatory mechanisms involved in melatonin biosynthesis in plants and investigate the capability of manipulating melatonin biosynthesis to enhance stress tolerance and growth in horticultural crops (Figure 1). The concept for drawing pathways has been taken [1][10]. Chemical structures were retrieved from PubChem.
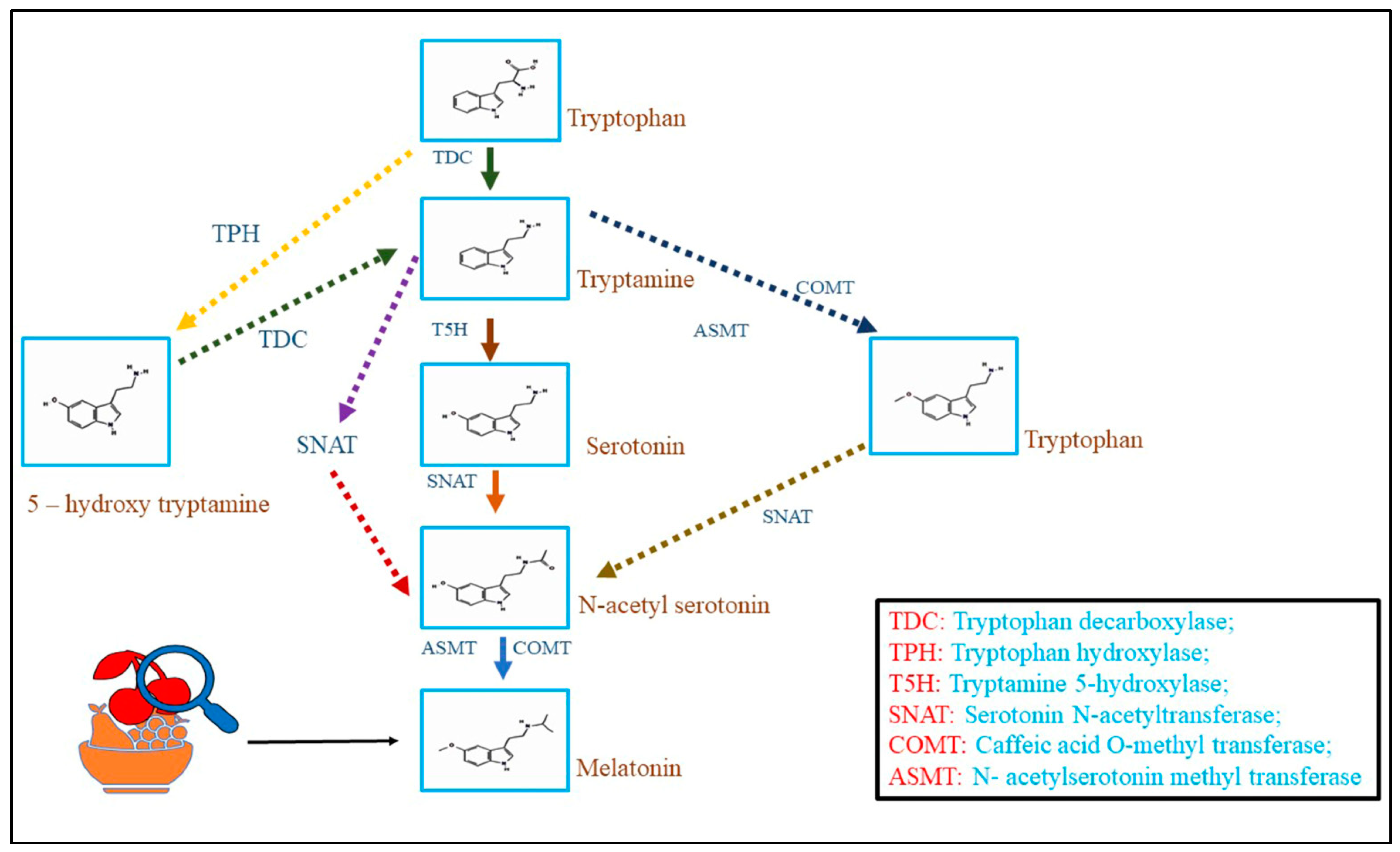
Figure 1.
Melatonin biosynthesis pathways in horticulture crops.
2. Methods of Analysis of Melatonin Level in Plants
Immersion, spray, soaking, and irrigation are the methods of applying melatonin in plants. Leaves, fruits, and above-ground plant parts can be suitable portions for applying exogenous melatonin hormone [104,105][11][12].
Melatonin analysis in plants can be conducted utilizing a variety of analytical techniques. Some of the most common ways are:
-
HPLC (high-performance liquid chromatography): This is a common technique for analyzing melatonin in plants. Melatonin is separated from other components of the plant extract depending on their chemical properties and detected using a UV detector. In this scenario, several types of detectors have been utilized, including an electrochemical detector, a fluorescence detector, and a UV detector. However, when compared to the MS detector, these detectors have revealed their limitations, specifically in terms of limited sensitivity and specificity. HPLC has been employed in measuring melatonin in a variety of plant species, including rice, grapevine, and tomato [16][8].
-
Gas chromatography–mass spectrometry (GC-MS): This technique uses gas chromatography to separate melatonin from other plant components and mass spectrometry to detect it. Although the sensitivity and specificity of melatonin quantification have improved, the need for sample volatile derivatization presents a disadvantage for its application to melatonin determination, as noted by [106][13] and [107][14]. Melatonin levels in plants such as wheat and barley have been measured using this method [7,16,108][8][15][16].
-
Enzyme-linked immunosorbent assay (ELISA): This is a recent technology that detects the presence of melatonin in plant extracts by using antibodies specific to melatonin. Melatonin quantification has been a long-standing practice using immunoassays such as radioimmunoassay (RIA) and enzyme-linked immunosorbent assay (ELISA). These methods have been studied by experts such as [109,[110,17111]][18][19]. The ELISA kit is widely available and is a commonly used tool for melatonin analysis due to its ease of use. However, antibody cross-reactivity is a concern that cannot be avoided, resulting in a decrease in specificity and accuracy [111,112][19][20]. Melatonin levels in plants such as soybean and grapevine have been measured using this method [109,113][17][21].
-
LC-MS/MS (liquid chromatography–tandem mass spectrometry): Melatonin is separated from other plant components using liquid chromatography and uses tandem mass spectrometry for detection, known for its sensitivity and selectivity. The combination of LC and MS has been established as a reliable and effective approach for conducting melatonin analysis due to its exceptional precision, reproducibility, and sensitivity [111][19]. Melatonin levels in plants such as tomatoes and kiwifruit have been measured using this method [16,109][8][17].
-
The method used to analyze plant melatonin is determined by sensitivity, selectivity, and sample complexity. To confirm the presence of melatonin and correctly quantify its levels, researchers frequently employ a combination of procedures.
3. Use of Exogenous Melatonin as a Potential Tool to Improve the Physiology of Horticultural Crops
When administered to horticultural crops, exogenous melatonin has been demonstrated to perform many physiological effects. It improves stress tolerance by lowering oxidative damage, regulating ion absorption and transport, and boosting root growth [24,30,35][22][23][24]. Melatonin therapy strategy, for example, has been shown to improve salt tolerance in tomato and cucumber plants by lowering oxidative damage and encouraging root growth [29,58][25][26]. Exogenous melatonin has also been shown to affect numerous aspects of horticultural crop growth and development, such as seed germination, root and shoot growth, and blooming [7,114][15][27]. It has been proven in enhancing seed development thereby promoting seed germination, facilitating root and shoot growth, and delaying senescence in various horticultural crops [58][26].
Exogenous melatonin has been reported to influence hormone signaling pathways in horticulture crops, thus regulating numerous physiological processes. Melatonin has been shown to interact with other plant hormones, such as auxins, cytokinins, and abscisic acid, in horticultural crops to increase root growth, postpone senescence, and improve stress tolerance [15,18,43][28][29][30]. Melatonin has also been found to serve as a broad-spectrum antioxidant in horticulture crops, scavenging ROS and boosting antioxidant enzyme activity [29,84][25][31].
In this way, exogenous melatonin has emerged as a potential tool for improving stress tolerance, growth, and development in horticultural crops. Its potential applications in crop production and stress management are increasingly being explored, and further research is necessary to fully understand the mechanisms of action and optimize its practical use in horticultural practices. The use of exogenous melatonin in alleviating abiotic stressors has been extensively studied by Ahmad et al. [6][32]. Melatonin has been found to be present in both roots and seeds, and its application has shown promising results in improving plant health. In general, when plants are subjected to sodium chloride (NaCl), plants typically suffer from decreased enzyme activity, resulting in salt sensitivity. However, exogenous melatonin application has been shown to increase the level of antioxidants and enzyme activity, thereby improving plant health. Additionally, melatonin has been found to increase chlorophyll content, chloroplast gene expression, and protein content, leading to increased photosynthetic activity and chlorophyll accumulation, which is critical to plant growth. Lastly, melatonin treatment during drought stress reduces ROS and O2− concentration while increasing chlorophyll content and physiological functions tied to plant development (Figure 2).
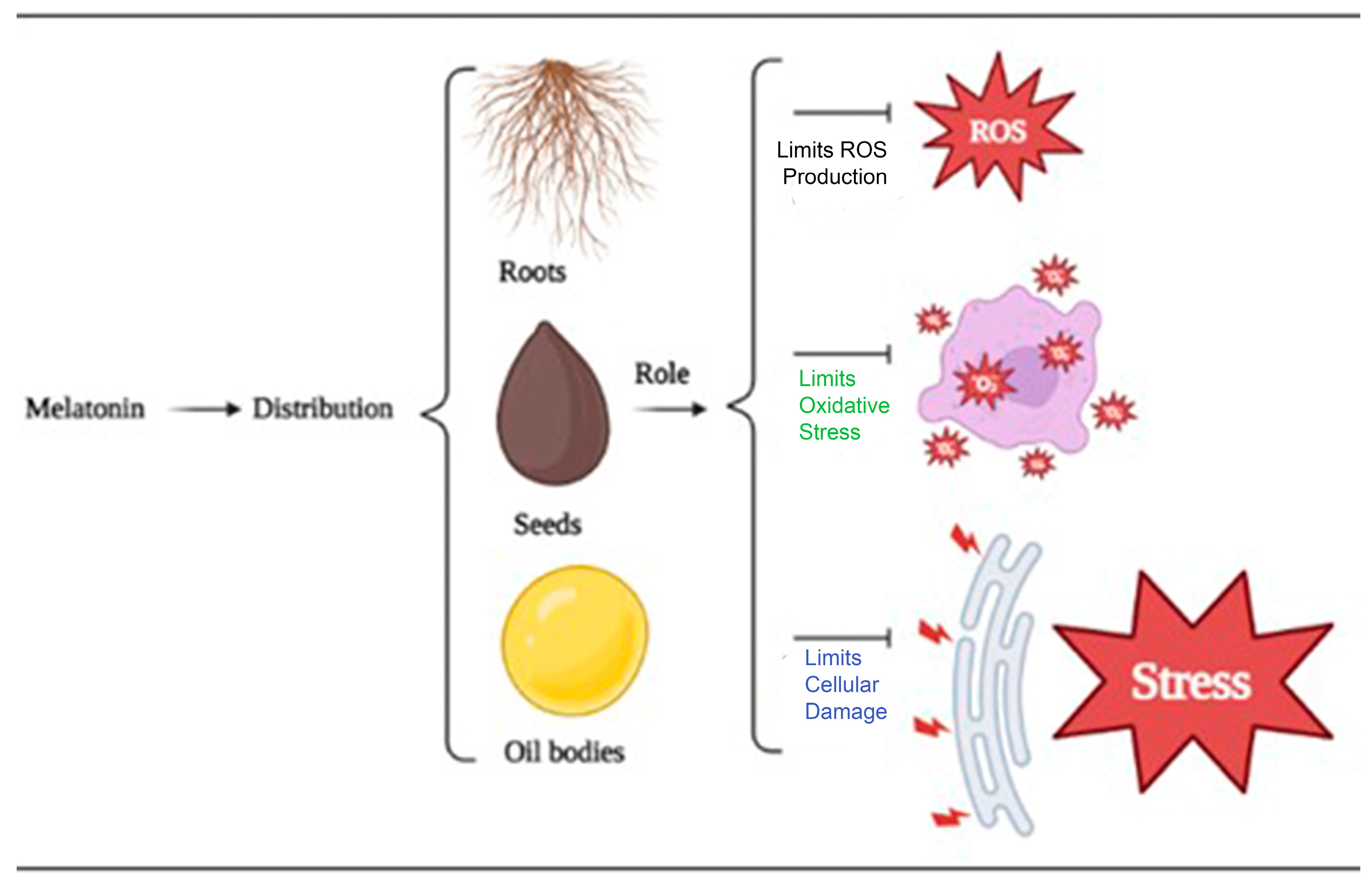
Figure 2.
The distribution and regulatory roles of an exogenous supply of melatonin in mitigating abiotic stresses.
It can be inferred that exogenous melatonin application has diverse effects on horticultural crops depending on the concentration and the stage of growth at which it is applied. Melatonin has been shown to promote seed germination, enhance plant growth and development, improve photosynthesis and antioxidant systems, increase fruit quality and storability, and mitigate the effects of abiotic stress factors such as drought, heat, salt, and cold. However, the optimal concentration of melatonin for each crop and growth stage needs to be further determined. Furthermore, the underlying molecular mechanisms of melatonin’s effects on horticultural crops require further research. Overall, exogenous melatonin application has enormous potential to improve the productivity and quality of horticultural crops, providing a sustainable and eco-friendly approach to crop management.
4. Economics for Using Melatonin in Horticultural Crops
The use of melatonin in horticultural crops has been shown to have potential economic benefits. Melatonin application can enhance crop growth and yield as well as improve quality attributes such as nutritional value, flavour, and shelf life. Melatonin application can increase profits for farmers and provide superior product value for consumers. One study evaluated the economic benefits of using melatonin in tomato production in China [38][1]. The study found that the application of melatonin at a concentration of 100 μM resulted in a 3.9% increase in tomato yield and an 18.7% increase in net photosynthesis compared to control treatments. Additionally, melatonin-treated tomatoes had higher levels of vitamin C, citric acid, lycopene, and soluble solids content, which could further enhance their market value. Similarly, a study on strawberry production found that melatonin application at a concentration of 100 μM resulted in a significant increase in yield and fruit quality, including a decline in fruit decay, extended shelf life, and enhanced accumulation of ATP and antioxidants [42][33]. The economic benefits of melatonin treatment were not directly evaluated in this restudyearch, but the improvements in yield and quality could translate into increased profitability for growers and improved product value for consumers.
Another study conducted on grapevines found that the application of melatonin at a concentration of 100 μM increased the accumulation of secondary metabolites such as anthocyanins and flavonoids, which contribute to the colour and flavour of grapes [115][34]. This could enhance the market value of the grapes and increase profitability for grape growers. Furthermore, the use of melatonin in the post-harvest storage of horticultural crops can also have economic benefits by lengthening their shelf life and minimizing post-harvest losses. For example, a study on post-harvest storage of strawberry and peach found that the application of melatonin at a concentration of 100 μM delayed fruit softening and reduced decay, resulting in longer shelf life and reduced post-harvest losses [42][33].
In addition to the direct economic benefits of melatonin application, there may also be indirect benefits such as reduced environmental impact and increased sustainability of horticultural production systems. Melatonin can enhance plant stress tolerance and reduce the need for chemical inputs such as fertilizers and pesticides, which can result in cost savings for growers and reduce the environmental impact of horticultural production. The use of melatonin in horticultural crops has the potential to provide economic benefits by improving crop growth, yield, and quality as well as reductions in post-harvest losses. However, the economic feasibility of melatonin application may depend on factors such as crop type, market demand, and the expense associated with melatonin treatment.
References
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.; Guo, S. Melatonin Pretreatment Confers Heat Tolerance and Repression of Heat-Induced Senescence in Tomato through the Modulation of ABA- and GA-Mediated Pathways. Front. Plant Sci. 2021, 12, 650955.
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin Regulates the Functional Components of Photosynthesis, Antioxidant System, Gene Expression, and Metabolic Pathways to Induce Drought Resistance in Grafted Carya cathayensis Plants. Sci. Total Environ. 2020, 713, 136675.
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.; Mittler, R.; Rivero, R. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535.
- Weaver, D.; Rivkees, S.; Reppert, S. Localization and Characterization of Melatonin Receptors in Rodent Brain by In Vitro Autoradiography. J. Neurosci. 1989, 9, 2581–2590.
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1177–1185.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207.
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin Receptor PMTR1-Mediated Signaling Regulates Stomatal Closure in Arabidopsis Thaliana. J. Pineal Res. 2018, 65, e12500.
- Chen, Q.; Qi, W.; Reiter, R.J.; Wei, W.; Wang, B. Exogenously Applied Melatonin Stimulates Root Growth and Raises Endogenous Indoleacetic Acid in Roots of Etiolated Seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328.
- Bychkov, I.A.; Andreeva, A.A.; Kudryakova, N.V.; Kusnetsov, V.V. Cytokinin Modulates Responses to Phytomelatonin in Arabidopsis thaliana under High Light Stress. Int. J. Mol. Sci. 2023, 24, 738.
- Gao, T.; Liu, X.; Tan, K.; Zhang, D.; Zhu, B.; Ma, F.; Li, C. Introducing Melatonin to the Horticultural Industry: Physiological Roles, Potential Applications, and Challenges. Hortic. Res. 2022, 9, uhac094.
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined Application of Arbuscular Mycorrhizal Fungi and Exogenous Melatonin Alleviates Drought Stress and Improves Plant Growth in Tobacco Seedlings. J. Plant Growth Regul. 2020, 40, 1074–1087.
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous Melatonin Treatment Induces Disease Resistance against Botrytis Cinerea on Post-Harvest Grapes by Activating Defence Responses. Foods 2022, 11, 2231.
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin Treatment Inhibits Gray Mold and Induces Disease Resistance in Cherry Tomato Fruit during Postharvest. Postharvest Biol. Technol. 2019, 157, 110962.
- Sheng, J.P.; Zhao, R.R.; Chen, L.L.; Shen, L. Effect of pre-harvest melatonin spraying on the post-harvest disease resistance and storage quality of tomato fruit. Food Sci. 2020, 141, 188–193.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends Plant Sci. 2014, 19, 789–797.
- Cao, J.J.; Yu, Z.C.; Zhang, Y.; Li, B.H.; Liang, W.X.; Wang, C.X. Control efficiency of exogenous melatonin against postharvest apple grey mould and its influence on the activity of defensive enzymes. Plant Physiol. 2017, 53, 1753–1760.
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2019, 10, 54.
- Zhao, L.; Chen, L.; Gu, P.; Zhan, X.; Zhang, Y.; Hou, C.; Wu, Z.; Wu, Y.-F.; Wang, Q.-C. Exogenous Application of Melatonin Improves Plant Resistance to Virus Infection. Plant Pathol. 2019, 68, 1287–1295.
- Afreen, F.; Zobayed, S.M.A.; Kozai, T. Melatonin in Glycyrrhiza uralensis: Response of Plant Roots to Spectral Quality of Light and UV-B Radiation. J. Pineal Res. 2006, 41, 108–115.
- Chen, Q.; Qi, W.; Li, M.; Wei, W. Melatonin in plants: Content, method and function. Chin. J. Appl. Environ. Biol. 2008, 14, 126–131.
- Badria, F.A. Melatonin, Serotonin, and Tryptamine in Some Egyptian Food and Medicinal Plants. J. Med. Food 2002, 5, 153–157.
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-Term Exogenous Application of Melatonin Delays Drought-Induced Leaf Senescence in Apple. J. Pineal Res. 2013, 54, 292–302.
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin Promotes Ripening and Improves Quality of Tomato Fruit during Postharvest Life. J. Exp. Bot. 2015, 66, 657–668.
- Guo, W.; Zhang, C.; Yang, R.; Zhao, S.; Han, X.; Wang, Z.; Li, S.; Gao, H. Endogenous Salicylic Acid Mediates Melatonin-Induced Chilling-and Oxidative-Stress Tolerance in Harvested Kiwifruit. Postharvest Biol. Technol. 2023, 201, 112341.
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin Promotes Seed Germination under High Salinity by Regulating Antioxidant Systems, ABA and GA4 Interaction in Cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279.
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin Applied to Cucumber (Cucumis sativus L.) Seeds Improves Germination during Chilling Stress. J. Pineal Res. 2009, 46, 214–223.
- Ragab, A.S.; Van Fleet, J.; Jankowski, B.; Park, J.-H.; Bobzin, S.C. Detection and Quantitation of Resveratrol in Tomato Fruit (Lycopersicon esculentum Mill.). J. Agric. Food Chem. 2006, 54, 7175–7179.
- Kumari, A.; Singh, S.K.; Mikhina, M.S.; Patni, B.; Krishna, G.K. Melatonin as a key regulator of plant growth and development. In Advancement of Melatonin Research in Plants: Multi-Faceted Role in Regulating Development and Stress Protection; Taylor & Francis Group: New York, NY, USA, 2023.
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting Roles of Melatonin in Adventitious Root Development of Solanum lycopersicum L. by Regulating Auxin and Nitric Oxide Signaling. Front. Plant Sci. 2016, 7, 718.
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin Treatment Delays Postharvest Senescence and Regulates Reactive Oxygen Species Metabolism in Peach Fruit. Postharvest Biol. Technol. 2016, 118, 103–110.
- Wang, P.; Sun, X.; Chang, C.; Feng, F.; Liang, D.; Cheng, L.; Ma, F. Delay in Leaf Senescence of Malus Hupehensis by Long-Term Melatonin Application Is Associated with Its Regulation of Metabolic Status and Protein Degradation. J. Pineal Res. 2013, 55, 424–434.
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The Role of Melatonin in Plant Growth and Metabolism, and Its Interplay with Nitric Oxide and Auxin in Plants under Different Types of Abiotic Stress. Front. Plant Sci. 2023, 14, 1108507.
- Dubocovich, M.L. Pharmacology and Function of Melatonin Receptors. FASEB J. 1988, 2, 2765–2773.
- Kolář, J.; Macháčková, I. Melatonin in Higher Plants: Occurrence and Possible Functions. J. Pineal Res. 2005, 39, 333–341.
More
