Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 3 by Catherine Yang.
Sirtuins belong to the class III histone deacetylases and possess nicotinamide adenine dinucleotide-dependent deacetylase activity. They are involved in the regulation of multiple signaling pathways implicated in cardiovascular diseases. Autophagy is a crucial adaptive cellular response to stress stimuli. Mounting evidence suggests a strong correlation between Sirtuins and autophagy, potentially involving cross-regulation and crosstalk. Sirtuin-mediated autophagy plays a crucial regulatory role in some cardiovascular diseases, including atherosclerosis, ischemia/reperfusion injury, hypertension, heart failure, diabetic cardiomyopathy, and drug-induced myocardial damage.
- Sirtuins
- autophagy
- FOXOs
- AMPK
- mTOR
- cardiovascular diseases
1. Atherosclerosis
AS is a chronic inflammatory disease characterized by elevated levels of LDL cholesterol in the plasma, endothelial dysfunction, inflammation, and immune cell infiltration. Sirtuins have been reported to directly affect AS formation and plaque stability by regulating cellular autophagy to prevent endothelial dysfunction, vascular smooth muscle cell senescence, and foam cell formation [1]. Foam cell formation is one of the key processes in the initial development of AS. Studies have elucidated that SIRT1 activation increases the expression of autophagy-associated proteins and the number of autophagosomes, promotes M2 macrophage polarization, reduces foam cell formation, and decreases plaque area and lipid accumulation, thereby delaying the progression of AS. The inhibition of SIRT1 abolishes these protective responses [2][3]. Inadequate or excessive activation of autophagy in endothelial progenitor cells (EPCs) can lead to endothelial dysfunction, while the restoration of autophagy or the inhibition of excessive autophagy in EPCs promotes vascular regeneration and repair, thereby exerting anti-AS effects [4][5]. Li et al. found that SIRT1 activation may delay AS development by inhibiting autophagy in EPCs through the Wnt/β-catenin/glycogen synthase kinase 3beta signaling pathway [6]. The release of thrombosis factors such as von Willebrand factor (vWF) and P-selectin plays a crucial role in AS and arterial thrombosis. The activation of the SIRT1/FOXO1 signaling pathway-mediated autophagy is a promising target for reducing vWF and P-selectin release and preventing AS [7]. Ma et al. found that inducing SIRT3/FOXO3a pathway-mediated mitophagy results in reduced plaque size and vulnerability, which ultimately alleviates inflammatory responses in AS [8]. Furthermore, SIRT6 overexpression significantly reduces foam cell formation by inducing autophagy in a macrophage foam cell model. Silencing of the key autophagy initiation gene ATG5 reverses the pro-autophagic effect of SIRT6, resulting in increased foam cell formation. Under conditions of ox-LDL, SIRT6 inhibits the expression of miR-33 and promotes autophagy and cholesterol efflux, thereby reducing foam cell formation in macrophages and attenuating the progression of AS. In contrast, knocking out the SIRT6 gene aggravates the formation of foam cells and the development of AS [9]. SIRT6 overexpression inhibits the expression of cell adhesion molecules in ox-LDL-treated mouse macrophages, leading to reduced macrophage and foam cell infiltration, significantly increasing macrophage autophagy flux, and thereby inhibiting macrophage apoptosis [10]. SIRT6-mediated autophagy is inhibited in endothelial cells treated with ox-LDL and high concentrations of glucose, whereas SIRT6 overexpression alleviates endothelial cell inflammation and reverses LDL endocytosis [11][12]. High shear stress leads to red blood cell destruction and iron deposition. Elevated iron levels in macrophages in plaques are associated with AS. The activation of SIRT1-mediated autophagy can inhibit the inflammatory response in excess iron autophagy foam cells with excess iron [13][14] (Figure 1).
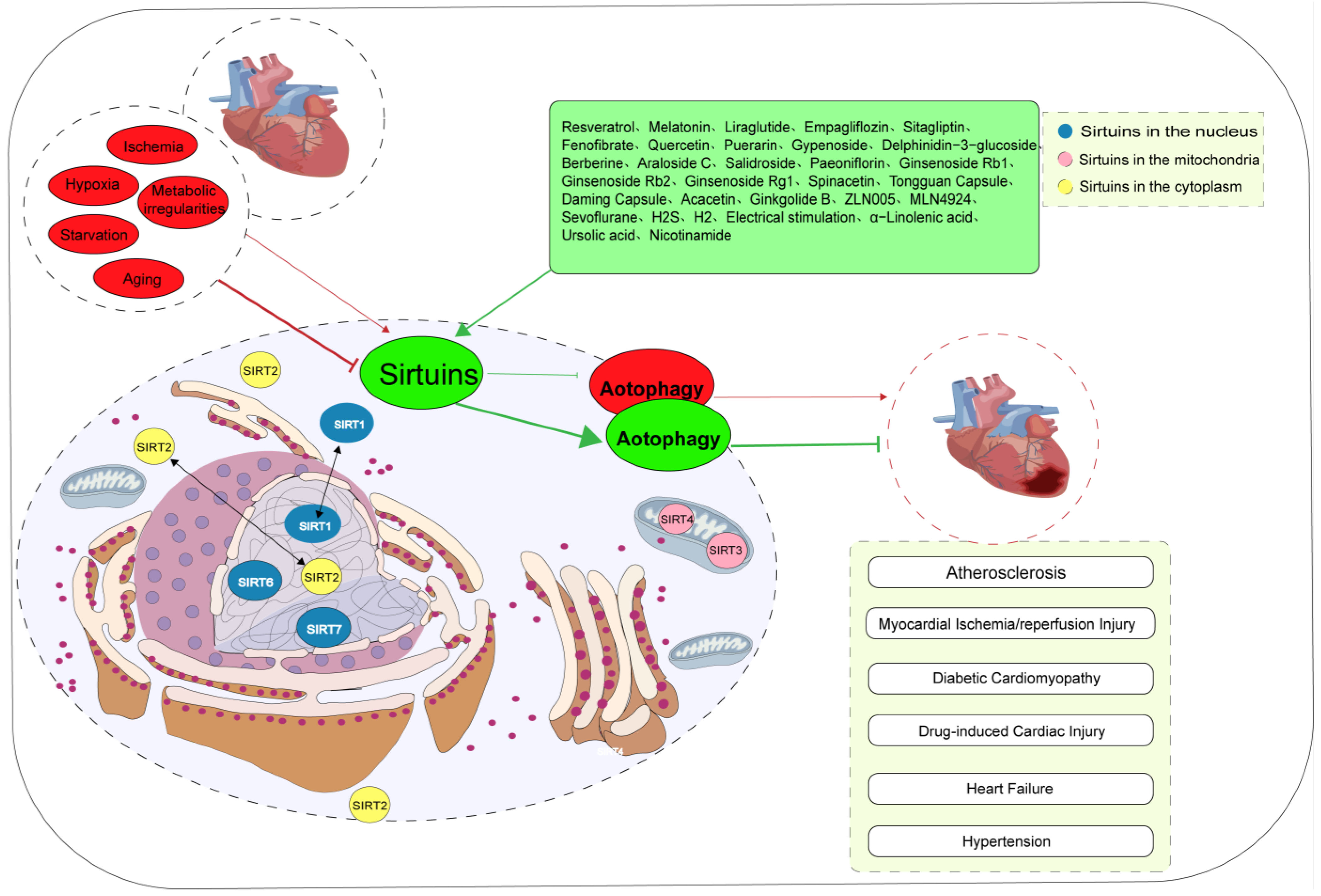
Figure 1. Biological significance of Sirtuin-mediated autophagy in cardiovascular diseases. The subcellular localization of Sirtuins proteins is contingent upon the specific cell type, cellular state, and molecular interactions. Most of the adverse factors, such as ischemia, hypoxia, starvation, metabolic abnormalities, and aging, lead to cardiovascular diseases by inhibiting Sirtuin-mediated autophagy. However, in a few cases, adverse factors contribute to the occurrence of cardiovascular disease by activating Sirtuin-mediated autophagy. At present, a variety of drugs can alleviate various stress injuries by regulating Sirtuin-mediated autophagy and having cardiovascular protective effects. SIRTs are silent information regulators.
2. Myocardial Ischemia/Reperfusion Injury
MI/R injury is a common pathophysiological process in cardiovascular diseases that often leads to reduced myocardium function, non-reflow phenomena, reperfusion arrhythmias, heart failure, and other problems. Factors leading to potential MI/R injury include free radical damage, calcium overload, inflammatory responses, oxidative stress, autophagy, apoptosis, ferroptosis and pyroptosis [15][16][17][18][19][20][21][22][23]. Presently, Sirtuin-mediated autophagy has been suggested to be involved in the process of the development of MI/R injury. In most cases, the activation of Sirtuin-mediated autophagy safeguards myocardial cells against MI/R injury. For example, the activation of the AMPK/SIRT1/FOXO1 and SIRT1/AMPK signaling pathways promotes autophagy, reduces oxidative stress in myocardial cells, significantly reduces myocardial infarct size, and improves heart function [24][25][26]. In addition, ROS generation is the most important signal mechanism in reperfusion-induced injury [27][28]. SIRT3-activated LKB1/AMPK and FOXO3a pathways in the ischemic phase induce the formation of autophagosomes and the activation of Parkin and PINK1, ultimately triggering mitophagy. However, because of the activation of abundant ROS during the reperfusion phase, SIRT3 downregulates the autophagy process by activating superoxide dismutase and eliminating ROS [24][29]. In vivo and in vitro experiments have shown that MI/R reduces SIRT3 expression and its deacetylase activity, which results in decreased antioxidant capacity and enhanced autophagy, whereas the upregulation of SIRT3 expression or ischemia pretreatment attenuates autophagic cell death, improves mitochondrial quality control, and reduces myocardial microvascular damage [30][31]. Recent studies have revealed that myocardin-related transcription factor A alleviates MI/R injury by inducing autophagy, and this protective effect is mediated by SIRT1-dependent autophagy [26]. In some cases, the activation of Sirtuin-mediated autophagy exacerbates MI/R injury. For instance, remote ischemic preconditioning activates SIRT3/hypoxia-inducible factor 1-α and inhibits autophagy, thereby exerting cardioprotective effects [32]. SIRT3 plays an essential role in refeeding syndrome-related myocardial injury during lipopolysaccharide-induced chronic sepsis in rats, possibly via the regulation of PINK/Parkin-mediated mitochondrial autophagy [33]. Low levels of SIRT6 expression have been found to be associated with increased all-cause mortality and notable adverse cardiovascular events in patients with acute myocardial infarction, and the activation of SIRT6-mediated autophagy has been shown to protect endothelial cells from post-ischemic inflammation [12]. Following acute cardiovascular injury in wild-type mice, SIRT7 expression increases, and SIRT7-deficient mice exhibit increased susceptibility to cardiac rupture following myocardial infarction, delayed blood flow restoration following ischemia, impaired wound healing following skin injury, reduced fibrosis and fibroblast differentiation, and decreased inflammatory cell infiltration in the infarct border zone. Additional studies have revealed that SIRT7 participates in tissue repair processes by regulating autophagy [34]. Nevertheless, a few studies suggest that the activation of Sirtuins may have detrimental effects on myocardial cells. For instance, thrombin exacerbates MI/R injury in myocardial cells by activating the SIRT1-mediated autophagy pathway [35] (Figure 1).
3. Diabetic Cardiomyopathy
DCM is a myocardial-specific microvascular complication in which autophagy is believed to play a dual role [36][37]. Autophagy in the heart of patients with diabetes is influenced by various factors, including blood glucose levels, obesity, insulin levels, glucose toxicity, lipid toxicity, oxidative stress, and inflammation. The activation of autophagy in the heart differs across distinct types of diabetes. For instance, cardiac autophagy is enhanced in type 1 diabetes mellitus but suppressed in type 2 diabetes mellitus [38]. Several studies have shown that the downregulation of SIRT1/SIRT3 contributes to DCM pathology through autophagy-related mechanisms [39][40][41]. For instance, high glucose levels lead to a decrease in the expression of SIRT1 and autophagy marker proteins, whereas the SIRT1 activator SRT1720 enhances autophagy [39]. Exposure of mice to streptozotocin causes myocardial injury and interstitial fibrosis, with increased apoptosis and mitochondrial damage in myocardial cells. In SIRT3-deficient mice, the effect of streptozotocin is more pronounced, and SIRT3 overexpression prevents mitochondrial damage and myocardial cell apoptosis. Further investigations have revealed that the downregulation of mitochondrial autophagy mediated by the SIRT3/FOXO3a/Parkin signaling pathway is a crucial process in the development of DCM [40]. Additionally, the inhibition of neuraminidase 1 activates AMPKα through LKB1, leading to SIRT3 activation, thereby modulating fibrosis, inflammation, apoptosis, and oxidative stress in cardiac tissue during DCM [41] (Figure 1).
4. Drug-Induced Cardiac Injury
DOX is an effective anthracycline chemotherapy drug, but its clinical application is limited owing to its cardiac toxicity. Autophagy plays a dual role in DOX-induced cardiac toxicity by inducing autophagy at low concentrations and inhibiting autophagy at high concentrations. Moderate levels of autophagy are crucial for organelle renewal and cell survival, whereas excessive activation of autophagy exacerbates cardiac toxicity. For example, experiments involving both in vivo and in vitro DOX-induced cardiac injury models have validated that acute high-dose DOX treatment suppresses AMPK and ULK1 activity, thereby impairing myocardial cell autophagy, whereas restoration of autophagy alleviates cardiac toxicity [42]. Conversely, autophagy has been shown to contribute to the apoptosis of cardiomyocytes and cardiac toxicity in a chronic DOX-induced cardiac toxicity model [43]. Research has shown that Sirtuins play a critical role in the regulation of autophagy during DOX-induced cardiac toxicity [44]. For instance, the activation of SIRT1 deacetylates TFEB and FOXOs, thereby promoting autophagolysosomal elimination and preventing DOX-induced cardiac toxicity [45]. Additionally, SIRT3 activates the mTOR/ULK1 pathway to inhibit NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation, restore mitochondrial autophagic flux, and alleviate cardiac toxicity [46]. Sunitinib, a novel anti-tumor drug, can cause hypertension, left ventricular systolic dysfunction, and myocardial cell death. SIRT3 overexpression inhibits autophagy and exacerbates cardiac toxicity, whereas restoration of autophagy by knocking out SIRT3 reverses the aforementioned cardiac toxicity [47]. Moreover, SIRT4 is closely associated with drug toxicity. For instance, in vivo and in vitro experiments have shown that SIRT4 expression improves cardiac function, reduces myocardial cell apoptosis and autophagy, and alleviates cardiac toxicity. However, the activation of mTOR eliminates the protective effect of SIRT4 overexpression on cardiac toxicity. Additional investigations have revealed that during DOX treatment, SIRT4 overexpression activates the AKT/mTOR signaling pathway, which in turn inhibits autophagy. These findings suggest that Sirtuins may serve as prospective targets for treating cardiac toxicity induced by anti-tumor drugs [48]. Currently, research on the involvement of SIRT5 in DOX-induced cardiac toxicity is limited; nevertheless, it is expected to acquire prominence as a critical field of study in the future in connection to drug-induced cardiac toxicity (Figure 1).
5. Heart Failure
Myocardial remodeling is a considerable pathological process in the development of chronic heart failure, characterized by myocardial hypertrophy, myocardial fibrosis, and apoptosis of myocardial cells. Multiple studies have suggested a potential link between the dysregulation of autophagy and the progression of myocardial remodeling [49]. The maintenance of normal function in myocardial cells relies heavily on basal levels of autophagy, and impaired autophagy leads to myocardial cell hypertrophy [50]. Insufficient autophagy during heart failure is associated with impaired SIRT1/PGC-1α and AMPK signaling, as well as the activation of the Akt/mTOR pathway. The upregulation of SIRT1, PGC-1α, and AMPK, along with inhibition of the Akt/mTOR pathway, promotes autophagy, diminishes myocardial hypertrophy, and improves heart failure [51][52]. The treatment of cardiac cells with Ang II results in a decrease in the expression of SIRT3 and autophagy-related proteins and an increase in the mRNA levels of atrial natriuretic peptide and B-type natriuretic peptide. The activation of SIRT3 promotes autophagy and alleviates Ang II-induced cardiac cell hypertrophy, whereas the silencing of SIRT3 exacerbates myocardial hypertrophy [53][54]. Omentin1 reduces myocardial hypertrophy by upregulating the SIRT3/FOXO3a signaling pathway, thereby initiating mitochondrial autophagy to maintain mitochondrial dynamic balance [55]. Endothelial-to-mesenchymal transition (EndoMT) is a critical pathological process in cardiac fibrosis. Studies have revealed that SIRT3 regulates autophagy-dependent glycolysis during EndoMT. SIRT3 deficiency reduces autophagy, whereas increased autophagy attenuates EndoMT [56]. Additionally, the positive regulation of autophagy by SIRT6 prevents isoproterenol-induced myocardial hypertrophy, possibly by attenuating Akt signaling and promoting accumulation of the FOXO3 transcription factor in the nucleus [57][58]. SIRT7 is involved in processes such as scar formation, angiogenesis, and inflammation. In cardiac fibroblasts, SIRT7 deficiency attenuates transforming growth factor-beta signaling and activates autophagy. This finding suggests that it plays a role in tissue repair by modulating autophagy [34]. Furthermore, SIRT3-deficient mice exhibit sparse cardiac microvasculature, functional hypoxia, impaired cardiac mitochondrial function, and cardiac fibrosis. SIRT3 overexpression restores angiogenic capacity, improves cardiac function, reduces fibrosis, and enhances PINK/Parkin-mediated mitochondrial autophagy, thereby alleviating mitochondrial dysfunction [59] (Figure 1).
6. Hypertension
A clinical study revealed a significant correlation between a polymorphism (rs2273773) of the SIRT1 gene and dynamic blood pressure levels in patients with hypertension of the Kazakh ethnic group, indicating a potential link between SIRT1 and blood pressure regulation [60]. Recent research has revealed contradictory roles for Sirtuin-mediated autophagy in the development of hypertension. For instance, exposure of rats to arsenic has been shown to significantly increase systolic blood pressure, impair contraction and relaxation responses in isolated aortas to Potassium chloride, Phenylephrine, and Acetylcholine, and upregulate the expression of SIRT1 and autophagy-related proteins [61], suggesting a negative effect of SIRT1-mediated autophagy on hypertension. However, in spontaneously hypertensive rats, SIRT3 expression is significantly decreased, and autophagy is significantly inhibited [62]. Limited studies have been conducted to investigate Sirtuin-mediated autophagy in hypertension. However, Sirtuins are expected to be candidate targets for the treatment of hypertension in the near future (Figure 1).
7. Cardiogenesis and Cardiac Maintenance
Cardiogenesis is a complex developmental process involving various overlapping stages of cell fate specification, proliferation, differentiation, and morphogenesis. SIRT1 exhibits significance not only in the early stages of embryogenesis but also in stages of cardiogenesis [63]. Li et al. showed that SIRT1 levels substantially decline in type II skeletal muscles, which notably exhibit marked atrophy. SIRT1 overexpression reduces muscle wastage by blocking the activation of FOXO1 and FOXO3 and by downregulating muscle-specific ubiquitin ligases, namely atrogin-1 and muscle RING-finger protein-1, along with multiple autophagy genes [64]. MiRNAs are involved in several core biological processes, including cardiogenesis, hematopoietic lineage differentiation, and oncogenesis. The expression of miR-199a is enhanced during the differentiation of pluripotent stem cells into endothelial cells. Notably, SIRT1 has been identified as a target of miR-199a [65]. Lin28, an RNA-binding protein, plays a role in the regulation of gene translation and is highly expressed in the early stages of embryogenesis. It is essential for modulating the self-renewal of stem cells. Lin28a can upregulate autophagy, inhibit cell damage, and maintain cell morphology and biological function subsequent to I/R injury by activating SIRT1/SIRT3 [66][67]. Consequently, SIRT1 and SIRT3 are compelling targets for cardiogenesis and cardiac maintenance (Figure 14).
References
- Grootaert, M.O.J.; Bennett, M.R. Sirtuins in atherosclerosis: Guardians of healthspan and therapeutic targets. Nat. Rev. Cardiol. 2022, 19, 668–683.
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 8, 2290–2307.
- Luo, Y.; Lu, S.; Gao, Y.; Yang, K.; Wu, D.; Xu, X.; Sun, G.; Sun, X. Araloside C attenuates atherosclerosis by modulating macrophage polarization via Sirt1-mediated autophagy. Aging 2020, 12, 1704–1724.
- Hassanpour, M.; Rezabakhsh, A.; Pezeshkian, M.; Rahbarghazi, R.; Nouri, M. Distinct role of autophagy on angiogenesis: Highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res. Ther. 2018, 9, 305.
- Wang, C.; Mao, C.; Lou, Y.; Xu, J.; Wang, Q.; Zhang, Z.; Tang, Q.; Zhang, X.; Xu, H.; Feng, Y. Monotropein promotes angiogenesis and inhibits oxidative stress-induced autophagy in endothelial progenitor cells to accelerate wound healing. J. Cell. Mol. Med. 2018, 22, 1583–1600.
- Li, Y.; Cui, W.; Song, B.; Ye, X.; Li, Z.; Lu, C. Autophagy-Sirtuin1(SIRT1) Alleviated the Coronary Atherosclerosis (AS)in Mice through Regulating the Proliferation and Migration of Endothelial Progenitor Cells (EPCs) via wnt/β-catenin/GSK3β Signaling Pathway. J. Nutr. Health Aging 2022, 26, 297–306.
- Wu, Q.; Hu, Y.; Jiang, M.; Wang, F.; Gong, G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4132.
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin Ameliorates the Progression of Atherosclerosis via Mitophagy Activation and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2018, 2018, 9286458.
- He, J.; Zhang, G.; Pang, Q.; Yu, C.; Xiong, J.; Zhu, J.; Chen, F. SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. FEBS J. 2017, 284, 1324–1337.
- Wang, T.; Sun, C.; Hu, L.; Gao, E.; Li, C.; Wang, H.; Sun, D. Sirt6 stabilizes atherosclerosis plaques by promoting macrophage autophagy and reducing contact with endothelial cells. Biochem. Cell Biol. 2020, 98, 120–129.
- Zhao, Y.; Jia, X.; Yang, X.; Bai, X.; Lu, Y.; Zhu, L.; Cheng, W.; Shu, M.; Zhu, Y.; Du, X.; et al. Deacetylation of Caveolin-1 by Sirt6 induces autophagy and retards high glucose-stimulated LDL transcytosis and atherosclerosis formation. Metabolism 2022, 131, 155162.
- Zi, Y.; Yi-An, Y.; Bing, J.; Yan, L.; Jing, T.; Chun-Yu, G.; Fan, P.; Hao, L.; Jia-Ni, T.; Han-Jin, H.; et al. Sirt6-induced autophagy restricted TREM-1-mediated pyroptosis in ox-LDL-treated endothelial cells: Relevance to prognostication of patients with acute myocardial infarction. Cell Death Discov. 2019, 5, 88.
- Yuan, P.; Hu, Q.; He, X.; Long, Y.; Song, X.; Wu, F.; He, Y.; Zhou, X. Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis. Cell Death Dis. 2020, 11, 141.
- Su, G.; Yang, W.; Wang, S.; Geng, C.; Guan, X. SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1Β and IL-18. Biochem. Biophys. Res. Commun. 2021, 561, 33–39.
- Garlick, P.B.; Davies, M.J.; Hearse, D.J.; Slater, T.F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ. Res. 1987, 61, 757–760.
- Liu, Y.; Zhang, J.; Zhang, D.; Yu, P.; Zhang, J.; Yu, S. Research Progress on the Role of Pyroptosis in Myocardial Ischemia-Reperfusion Injury. Cells 2022, 11, 3271.
- Koltai, M.; Tosaki, A.; Hosford, D.; Braquet, P. Ginkgolide B protects isolated hearts against arrhythmias induced by ischemia but not reperfusion. Eur. J. Pharmacol. 1989, 164, 293–302.
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1553–H1568.
- Sinning, C.; Westermann, D.; Clemmensen, P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark. Med. 2017, 11, 11031–11040.
- Morales, C.R.; Pedrozo, Z.; Lavandero, S.; Hill, J.A. Oxidative stress and autophagy in cardiovascular homeostasis. Antioxid. Redox Signal. 2014, 20, 507–518.
- Chen-Scarabelli, C.; Agrawal, P.R.; Saravolatz, L.; Abuniat, C.; Scarabelli, G.; Stephanou, A.; Loomba, L.; Narula, J.; Scarabelli, T.M.; Knight, R. The role and modulation of autophagy in experimental models of myocardial ischemia-reperfusion injury. J. Geriatr. Cardiol. 2014, 11, 338–348.
- Lazou, A.; Iliodromitis, E.K.; Cieslak, D.; Voskarides, K.; Mousikos, S.; Bofilis, E.; Kremastinos, D.T. Ischemic but not mechanical preconditioning attenuates ischemia/reperfusion induced myocardial apoptosis in anaesthetized rabbits: The role of Bcl-2 family proteins and ERK1/2. Apoptosis 2006, 11, 2195–2204.
- Yan, H.F.; Tuo, Q.Z.; Yin, Q.Z.; Lei, P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool. Res. 2020, 41, 220–230.
- Li, H.; Zheng, F.; Zhang, Y.; Sun, J.; Gao, F.; Shi, G. Resveratrol, novel application by preconditioning to attenuate myocardial ischemia/reperfusion injury in mice through regulate AMPK pathway and autophagy level. J. Cell. Mol. Med. 2022, 26, 4216–4229.
- Luo, G.; Jian, Z.; Zhu, Y.; Zhu, Y.; Chen, B.; Ma, R.; Tang, F.; Xiao, Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int. J. Mol. Med. 2019, 43, 2033–2043.
- Zhong, Z.; Luo, X.Y.; Xiang, P.; Ji, H.H.; Wu, X.D.; Chong, A.G.; Hu, X.Y.; Cao, X.L. MRTF-A alleviates myocardial ischemia reperfusion injury by inhibiting the inflammatory response and inducing autophagy. Mol. Cell. Biochem. 2023, 478, 343–359.
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407.
- Blasig, I.E.; Ebert, B.; Hennig, C.; Pali, T.; Tosaki, A. Inverse relationship between ESR spin trapping of oxyradicals and degree of functional recovery during myocardial reperfusion in isolated working rat heart. Cardiovasc. Res. 1990, 24, 263–270.
- Zheng, Y.; Shi, B.; Ma, M.; Wu, X.; Lin, X. The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J. Cell. Physiol. 2019, 234, 5488–5495.
- Wu, D.; Ji, H.; Du, W.; Ren, L.; Qian, G. Mitophagy alleviates ischemia/reperfusion-induced microvascular damage through improving mitochondrial quality control. Bioengineered 2022, 13, 3596–3607.
- Ma, L.L.; Kong, F.J.; Dong, Z.; Xin, K.Y.; Wang, X.X.; Sun, A.J.; Zou, Y.Z.; Ge, J.B. Hypertrophic Preconditioning Attenuates Myocardial Ischaemia-Reperfusion Injury by Modulating SIRT3-SOD2-mROS-Dependent Autophagy. Cell Prolif. 2021, 54, e13051.
- Gao, R.; Lv, C.; Qu, Y.; Yang, H.; Hao, C.; Sun, X.; Hu, X.; Yang, Y.; Tang, Y. Remote Ischemic Conditioning Mediates Cardio-protection After Myocardial Ischemia/Reperfusion Injury by Reducing 4-HNE Levels and Regulating Autophagy via the ALDH2/SIRT3/HIF1α Signaling Pathway. J. Cardiovasc. Transl. Res. 2023, 1–14.
- Li, J.; Lu, K.; Zhang, X.; Wang, T.; Li, Q.; Yu, X.; Han, W.; Sun, L. SIRT3-mediated mitochondrial autophagy in refeeding syndrome-related myocardial injury in sepsis rats. Ann. Transl. Med. 2022, 10, 211.
- Araki, S.; Izumiya, Y.; Rokutanda, T.; Ianni, A.; Hanatani, S.; Kimura, Y.; Onoue, Y.; Senokuchi, T.; Yoshizawa, T.; Yasuda, O.; et al. Sirt7 Contributes to Myocardial Tissue Repair by Maintaining Transforming Growth Factor-β Signaling Pathway. Circulation 2015, 132, 1081–1093.
- Wang, X.; Xu, Y.; Li, L.; Lu, W. Thrombin Aggravates Hypoxia/Reoxygenation Injury of Cardiomyocytes by Activating an Autophagy Pathway-Mediated by SIRT1. Med. Sci. Monit. 2021, 27, e928480.
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638.
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338.
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Mikami, A.; Ogino, A.; Watanabe, T.; Morishita, K.; Okada, H.; Kawasaki, M.; et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 2015, 11, 1146–1160.
- Qiu, Z.; Ming, H.; Zhang, Y.; Yu, Y.; Lei, S.; Xia, Z.Y. The Protective Role of Bmal1-Regulated Autophagy Mediated by HDAC3/SIRT1 Pathway in Myocardial Ischemia/Reperfusion Injury of Diabetic Rats. Cardiovasc. Drugs Ther. 2022, 36, 229–243.
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 1973–1983.
- Guo, Z.; Tuo, H.; Tang, N.; Liu, F.Y.; Ma, S.Q.; An, P.; Yang, D.; Wang, M.Y.; Fan, D.; Yang, Z.; et al. Neuraminidase 1 deficiency attenuates cardiac dysfunction, oxidative stress, fibrosis, inflammatory via AMPK-SIRT3 pathway in diabetic cardiomyopathy mice. Int. J. Biol. Sci. 2022, 18, 826–840.
- Kawaguchi, T.; Takemura, G.; Kanamori, H.; Takeyama, T.; Watanabe, T.; Morishita, K.; Ogino, A.; Tsujimoto, A.; Goto, K.; Maruyama, R.; et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc. Res. 2012, 96, 456–465.
- Sishi, B.J.; Loos, B.; van Rooyen, J.; Engelbrecht, A.M. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem. Pharmacol. 2013, 85, 124–134.
- Govender, J.; Loos, B.; Marais, E.; Engelbrecht, A.M. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: A review of the protective role of melatonin. J. Pineal Res. 2014, 57, 367–380.
- Zheng, D.; Zhang, Y.; Zheng, M.; Cao, T.; Wang, G.; Zhang, L.; Ni, R.; Brockman, J.; Zhong, H.; Fan, G.C.; et al. Nicotinamide riboside promotes autolysosome clearance in preventing doxorubicin-induced cardiotoxicity. Clin. Sci. 2019, 133, 1505–1521.
- Sun, Z.; Fang, C.; Xu, S.; Wang, B.; Li, D.; Liu, X.; Mi, Y.; Guo, H.; Jiang, J. SIRT3 attenuates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome via autophagy. Biochem. Pharmacol. 2023, 207, 115354.
- Yang, Y.; Li, N.; Chen, T.; Zhang, C.; Li, J.; Liu, L.; Qi, Y.; Zheng, X.; Zhang, C.; Bu, P. Sirt3 promotes sensitivity to sunitinib-induced cardiotoxicity via inhibition of GTSP1/JNK/autophagy pathway in vivo and in vitro. Arch. Toxicol. 2019, 93, 3249–3260.
- He, L.; Wang, J.; Yang, Y.; Zou, P.; Xia, Z.; Li, J. SIRT4 Suppresses Doxorubicin-Induced Cardiotoxicity by Regulating the AKT/mTOR/Autophagy Pathway. Toxicology 2022, 469, 153119.
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation 2016, 133, 1249–1263.
- Zheng, C.B.; Gao, W.C.; Xie, M.; Li, Z.; Ma, X.; Song, W.; Luo, D.; Huang, Y.; Yang, J.; Zhang, P.; et al. Ang II Promotes Cardiac Autophagy and Hypertrophy via Orai1/STIM1. Front. Pharmacol. 2021, 12, 622774.
- Packer, M. Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: Implications for understanding the effects of current and future treatments for heart failure. Eur. Heart J. 2020, 41, 3856–3861.
- Jiang, X.; Zhang, K.; Gao, C.; Ma, W.; Liu, M.; Guo, X.; Bao, G.; Han, B.; Hu, H.; Zhao, Z. Activation of FMS-like tyrosine kinase 3 protects against isoprenaline-induced cardiac hypertrophy by improving autophagy and mitochondrial dynamics. FASEB J. 2022, 36, e22672.
- Wang, H.N.; Li, J.L.; Xu, T.; Yao, H.Q.; Chen, G.H.; Hu, J. Effects of Sirt3-autophagy and resveratrol activation on myocardial hypertrophy and energy metabolism. Mol. Med. Rep. 2020, 22, 1342–1350.
- Li, J.; Chen, T.; Xiao, M.; Li, N.; Wang, S.; Su, H.; Guo, X.; Liu, H.; Yan, F.; Yang, Y.; et al. Mouse Sirt3 promotes autophagy in AngII-induced myocardial hypertrophy through the deacetylation of FoxO1. Oncotarget 2016, 7, 86648–86659.
- Hu, J.; Liu, T.; Fu, F.; Cui, Z.; Lai, Q.; Zhang, Y.; Yu, B.; Liu, F.; Kou, J.; Li, F. Omentin1 ameliorates myocardial ischemia-induced heart failure via SIRT3/FOXO3a-dependent mitochondrial dynamical homeostasis and mitophagy. J. Transl. Med. 2022, 20, 447.
- Gao, J.; Wei, T.; Huang, C.; Sun, M.; Shen, W. Sirtuin 3 governs autophagy-dependent glycolysis during Angiotensin II-induced endothelial-to-mesenchymal transition. FASEB J. 2020, 34, 16645–16661.
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650.
- Lu, J.; Sun, D.; Liu, Z.; Li, M.; Hong, H.; Liu, C.; Gao, S.; Li, H.; Cai, Y.; Chen, S.; et al. SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl. Res. 2016, 172, 96–112.e6.
- Wei, T.; Huang, G.; Gao, J.; Huang, C.; Sun, M.; Wu, J.; Bu, J.; Shen, W. Sirtuin 3 Deficiency Accelerates Hypertensive Cardiac Remodeling by Impairing Angiogenesis. J. Am. Heart Assoc. 2017, 6, e006114.
- Zhong, X.L.; Miao, H.J.; Fang, Z.M.; Kuken, B.; Song, H.Y.; Zhong, H.; Lu, Y.; Liu, S.M. The effect of SIRT1 gene polymorphisms on ambulatory blood pressure of hypertensive patients in the Kazakh population. Genet. Test. Mol. Biomark. 2015, 19, 561–565.
- Balarastaghi, S.; Barangi, S.; Hosseinzadeh, H.; Imenshahidi, M.; Moosavi, Z.; Razavi, B.M.; Karimi, G. Melatonin improves arsenic-induced hypertension through the inactivation of the Sirt1/autophagy pathway in rat. Biomed. Pharmacother. 2022, 151, 113135.
- Li, G.; Wang, X.; Yang, H.; Zhang, P.; Wu, F.; Li, Y.; Zhou, Y.; Zhang, X.; Ma, H.; Zhang, W.; et al. α-Linolenic acid but not linolenic acid protects against hypertension: Critical role of SIRT3 and autophagic flux. Cell Death Dis. 2020, 11, 83.
- Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004, 556, 281–286.
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526.
- Li, Z.; Margariti, A.; Wu, Y.; Yang, F.; Hu, J.; Zhang, L.; Chen, T. MicroRNA-199a induces differentiation of induced pluripotent stem cells into endothelial cells by targeting sirtuin 1. Mol. Med. Rep. 2015, 12, 3711–3717.
- Chen, D.; Zheng, K.; Wu, H.; Zhang, X.; Ye, W.; Tan, X.; Xiong, Y. Lin28a attenuates cerebral ischemia/reperfusion injury through regulating Sirt3-induced autophagy. Brain Res. Bull. 2021, 170, 39–48.
- Hao, Y.; Lu, Q.; Yang, G.; Ma, A. Lin28a protects against postinfarction myocardial remodeling and dysfunction through Sirt1 activation and autophagy enhancement. Biochem. Biophys. Res. Commun. 2016, 479, 833–840.
More
