Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Tomislav Cengic.
Bone morphogenesis (osteogenesis) is the process of formation and maintenance of bone tissue and is the result of bone formation and bone resorption. Both processes, bone formation (osteoproduction) and bone resorption (osteoresorption), are functionally balanced in the creation and maintenance of optimal functional structure, or homeostasis, of the skeletal system according to functional demands. Deviation from the physiological balance of these processes is manifested in pathological osteogenesis. One morphological substrate of pathological osteogenesis is osteophyte formation in osteoarthritis (OA).
- osteoarthritis
- osteophyte
- bone remodeling
1. Osteophytes Morphology and Development
Roland and Moskowitz consider osteoarthritis (OA) as the final stage of heterogeneous etiopathogenetic events affecting the joints [37][1]. In joints affected by OA changes, in addition to disturbances in the structure of the articular cartilage and subchondral bone tissue, osteophytes develop [38][2]. Osteophytes are bony outgrowths covered with a fibrocartilaginous cap (from Greek “osteo” = bone + “phyton” = plant, vegetation). Genuine osteophytes (osteochondrophytes) grow under the periosteum, along the edge of the articular cartilage or the insertion of the synovial membrane. In this regard, osteochondrophytes are called marginal osteophytes. The position of marginal osteophytes corresponds to the area where three different joint structures come into contact (articular cartilage, synovial membrane, and periosteum). The synovial fluid enables an additional indirect relationship between these tissues. Marginal osteophytes are a common finding in degenerative OA.
In addition to genuine osteophytes, traction and inflammatory osteophytes have been described. Traction osteophytes develop in the areas of tendon insertions (enthesophytes), while inflammatory osteophytes (syndesmophytes) are characteristic of ankylosing spondylitis. Osteophytes that appear further from the edge of the articular cartilage are called central osteophytes. Their position corresponds to the areas of damaged articular cartilage.
Osteophytes are clearly visible on X-ray images of joints affected by OA. Their distribution and dimensions can be precisely analyzed on appropriate joint radiographs.
The cause that triggers the development of osteophytes has not been established. The development of osteophytes involves morphogenetic processes from the early stages of skeletal development (endochondral and intramembranous ossification). Cytomorphologically and according to gene expression patterns, chondrogenesis and the formation of new bone tissue during osteophyte development particularly resemble identical processes in fracture healing with callus formation [39][3]. Possible causes of osteophyte development include mechanical overload of the joint and humoral factors. Experimental models of OA are based on these factors, such as cruciate ligament transection in the knee joint (biomechanical) and the administration of TGF-β1 and bone morphogenetic protein 2 (BMP-2) (humoral) into the joint cavity [40,41][4][5]. Destruction of the articular cartilage and the development of osteophytes have also been found in biomechanically unloaded joints during immobilization [42][6]. Therefore, previous notions that osteoarthritis is a causal consequence of aging have been rejected because cartilage atrophy alone does not always result in osteoarthritis. BMPs are a subset of the TGF-β superfamily of signaling molecules, and play a crucial role in regulating various cellular processes, particularly in the development and maintenance of bones, cartilage, and other tissues. BMP-2 promotes bone formation by stimulating the differentiation of mesenchymal stem cells into osteoblasts, facilitating the creation of new bone tissue [43,44][7][8].
Since the cause of osteophyte development has not been determined, despite the existence of experimental models, the question arises whether it is a functional adaptation of the joint or a pathological change. The answer still relies on assumptions. One theory suggests that osteophytes are the organism’s attempt to stabilize a biomechanically insufficient joint by increasing the surface area of the articular cartilage [45][9]. As OA progresses and articular cartilage degenerates, it is believed that osteophytes represent an adaptive attempt by the organism to repair the degenerated cartilage rather than a degenerative change [46][10]. It is probable that the local humoral milieu, established through certain biomechanical stimuli, stimulates chondrogenesis and endochondral ossification, leading to osteophyte formation [45][9].
In animal experimental models of OA, stages of osteophyte development have been described. The dominant mechanism of osteophyte formation is endochondral ossification, in which osteogenic cells mainly originate from the periosteum. Areas of intramembranous ossification appear during the definitive shaping of osteophytes, where osteogenic cells mainly originate from the synovial membrane. The expression of numerous growth factors has been investigated in osteophytes [45][9]. Research results regarding the expression of bone morphogenetic proteins in osteophytes are ambiguous. In rat periosteal stem cells, TGF-β did not induce chondrogenesis, while treatment of these cells with BMP-2 resulted in the production of collagen type II and aggrecan. Chondrocytes treated with BMP-2 showed markers of chondrocyte hypertrophy (collagen type X, osteocalcin), while in the culture treated with a combination of BMP-2 and TGF-β, chondrocyte hypertrophy was absent [47][11]. However, Uuistalo et al. showed that BMP-2 stimulates chondrogenesis from mouse periosteal stem cells and supports further endochondral ossification [48][12]. In a study on avian species, BMP-2 did not exhibit a chondrogenic effect on periosteal cells [49][13]. Chondrogenic differentiation of human mesenchymal stem cells from the synovial membrane was observed after the addition of TGF-β to the culture. Joyce et al., studying periosteal ossification in rats, found that depending on the dosage, TGF-β directs ossification in an endochondral or intramembranous direction. High doses of exogenous TGF-β introduced into the periosteum support endochondral ossification, while at lower doses, ossification mainly occurs intramembranously [50,51][14][15].
2. Radiological Characteristics of Osteoarthritis
It is believed that changes in subchondral bone tissue play a crucial role in the pathogenesis of osteoarthritis and can be detected early, long before clinical signs appear [52,53][16][17]. In the early stages of osteoarthritis, radiological signs are sparse, so depending on the stage of osteoarthritis, it is necessary to choose the appropriate radiological examination method. The expression of the radiological signs depends on the biomechanical characteristics of the affected joint.
As degenerative changes progress, the articular cartilage deteriorates, and the joint space decreases, which is evident on X-rays as a narrowing of the joint space. The joint surfaces become deformed, flattened, uneven, or irregularly shaped. The latter is the result of focal cartilage proliferation that calcifies, leading to double or triple contour deformities on X-rays (Figure 1). Subchondral sclerosis of the joint surfaces can progress to eburnation (ivory-like appearance). Convex joint bodies lose their convexity, while concave joint bodies become shallower and flattened. Hypertrophic changes in the form of osteophytes develop chronically along the edges of the joint surfaces. Increasing osteophytes can result in a bony bridging between the articulating joint surfaces. Bone pseudocysts appear as cyst-like, marginally sclerotic transparencies in the subchondral area, varying in size and shape. Enlarged pseudocysts can cause subluxation of the joint bodies and the presence of loose bone fragments, called joint mice, in the joint space. Fracture of the bony wall of a pseudocyst can create communication between the joint cavity and the denuded joint body. Since 1957, the Kellgren and Lawrence classification has been used for the assessment of osteoarthritis changes in radiological practice. The radiological analysis includes osteophytes, periarticular ossifications, changes in articular cartilage associated with subchondral sclerosis of bone tissue, and pseudocysts in the subchondral bone tissue. According to the radiological findings, osteoarthritis changes are graded into five stages (0–4) [54][18].
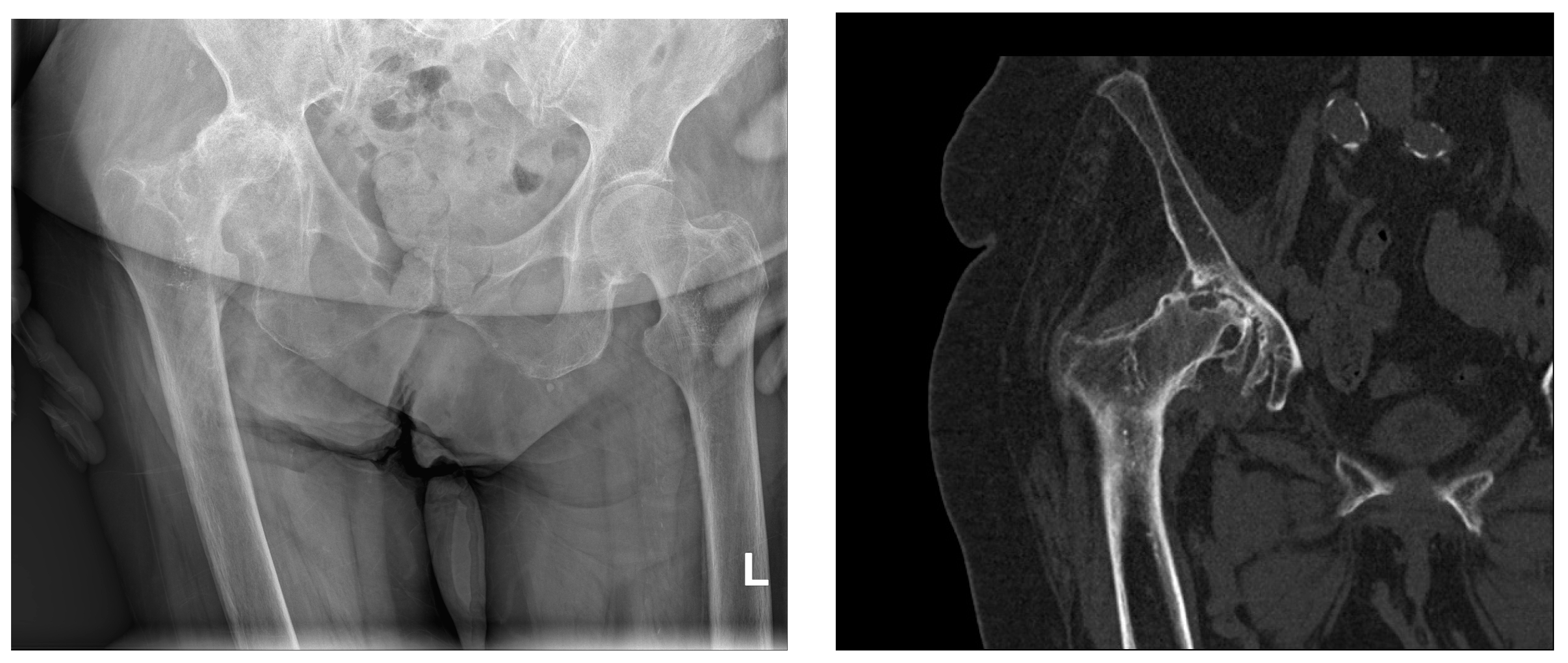
Figure 1. Sagittal X-ray of the pelvis and computerized tomography of the right hip (same patient). Severe right-sided coxarthrosis deformans. Grossly reduced joint space. Dense, sclerotic subchondral bone tissue of the deformed articular bodies interspersed with cyst-like transparencies of bone. Abundant marginal osteophytes of the articular surfaces and femoral head subluxation with consequent higher position of the right half of the pelvis. L—left side.
Complete joint ankylosis as a result of osteoarthritis is rarely seen today. Cartilaginous elements in some joints often become calcified (chondrocalcinosis which should be differentiated from calcium pyrophosphate dihydrate deposition disease), such as the menisci in the knee joint or the glenoid labrum in the shoulder joint. The radiographic findings often do not correspond to the subjective complaints of the patients, so in the presence of a rich physical examination and clinical picture, the radiographic findings may be only minimally altered. Computed tomography, compared to conventional radiographic imaging of joints affected by osteoarthritis, provides richer data on the extent of the pathoanatomical substrate.
In early osteoarthritis changes, visible on magnetic resonance imaging (MRI), there are subchondral sclerosis and cysts in the bone tissue, lesions in the surrounding bone marrow, fissures in the articular cartilage (Figure 2), and changes in the synovial membrane [55][19].
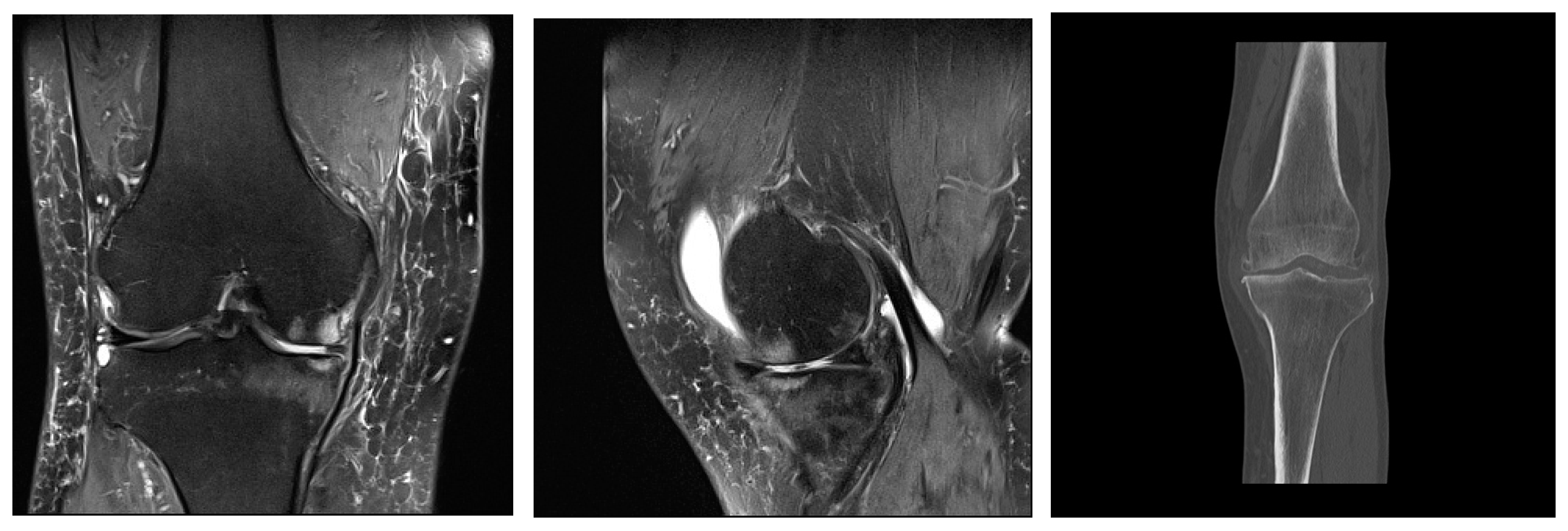
Figure 2. Mild deforming gonarthrosis visualized by MRI (proton-density fat saturated sequences in sagittal and coronal plane) and by CT (multiplanar reconstruction, coronal plane). Patchy areas of subchondral bone edema of femoral and tibial articulations with numerous marginal osteophytes. Articular cartilage is denuded in medial compartment, while in lateral compartment I-II degree hondromalacic changes are seen. Narrowed articular spaces with reactive effusion propagating to suprapatellar bursa are seen. High signal intensity of menisci and cruciate ligaments suggestive of degenerative changes. Ruptured medial meniscus. Reactive edema of periarticular and subcutaneous soft tissues.
The role of ultrasound examination of the joints primarily relates to non-skeletal tissues (periosteum, synovial membrane, tendons, ligaments, etc.). Ultrasound can differentiate marginal bone appositions on joint bodies (osteophytes) and the width of the joint space, as well as its expansion due to effusion (Figure 3). Some components of the accessory structure of the joint, such as the meniscus in the knee joint, can be partially visualized with ultrasound (Figure 4). Synovial bursae adjacent to joints affected by osteoarthritis serve as an anatomical substrate for the development of cysts that can be well examined by ultrasound. Examples include the bursae in the popliteal fossa (bursae mucosae regionis genus posterioris), among which the bursa musculi semimembranosi and the bursa subtendinea musculi gastrocnemii medialis, often combined into one, from which a Baker’s cyst develops in 10–20% of cases of knee osteoarthritis. When a Baker’s cyst communicates with the joint space of the knee joint, inflammatory processes from the cyst can directly transfer to the knee joint (Figure 5).
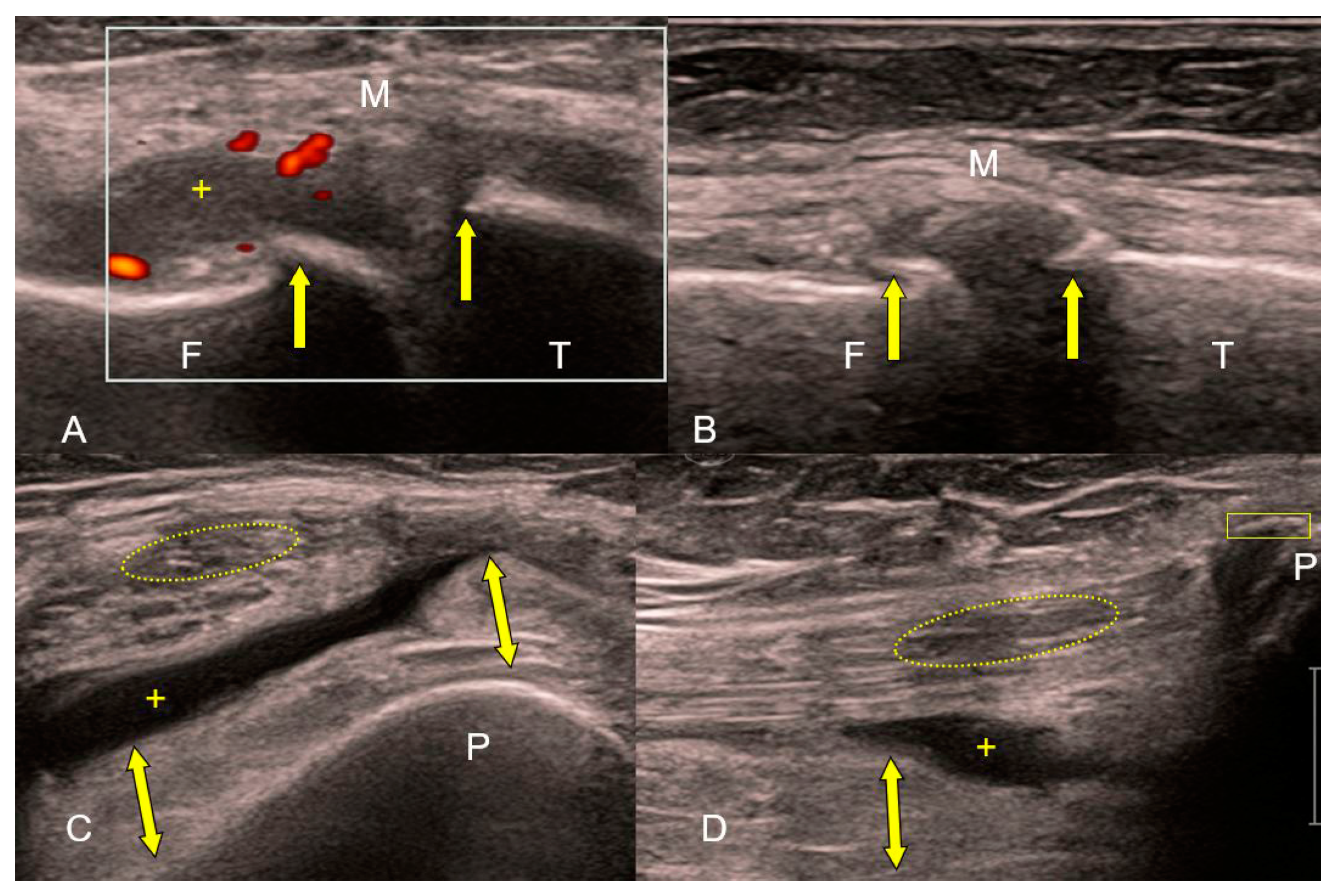
Figure 3. (A–D) Ultrasound of the knee joint in exacerbation of osteoarthritis (high-frequency linear probe: B-mode and Power Doppler). Irregular echoes from the femoral (F) and tibial (T) articular surfaces corresponding to marginal osteophytes (A,B). Hyperemia (red color in white rectangle) and edema of the thickened, hypertrophic synovial membrane (yellow bidirectional arrows) with effusion (+) in the suprapatellar bursa are signs of reactive inflammation. Loss of tension and fine linear echostructure of the medial collateral ligament (M). Absence of linear echostructure and decreased echogenicity (yellow ellipse) in the distal part of the quadriceps muscle tendon. Enthesophyte (yellow rectangle) at the base of the patella (P) (C,D).
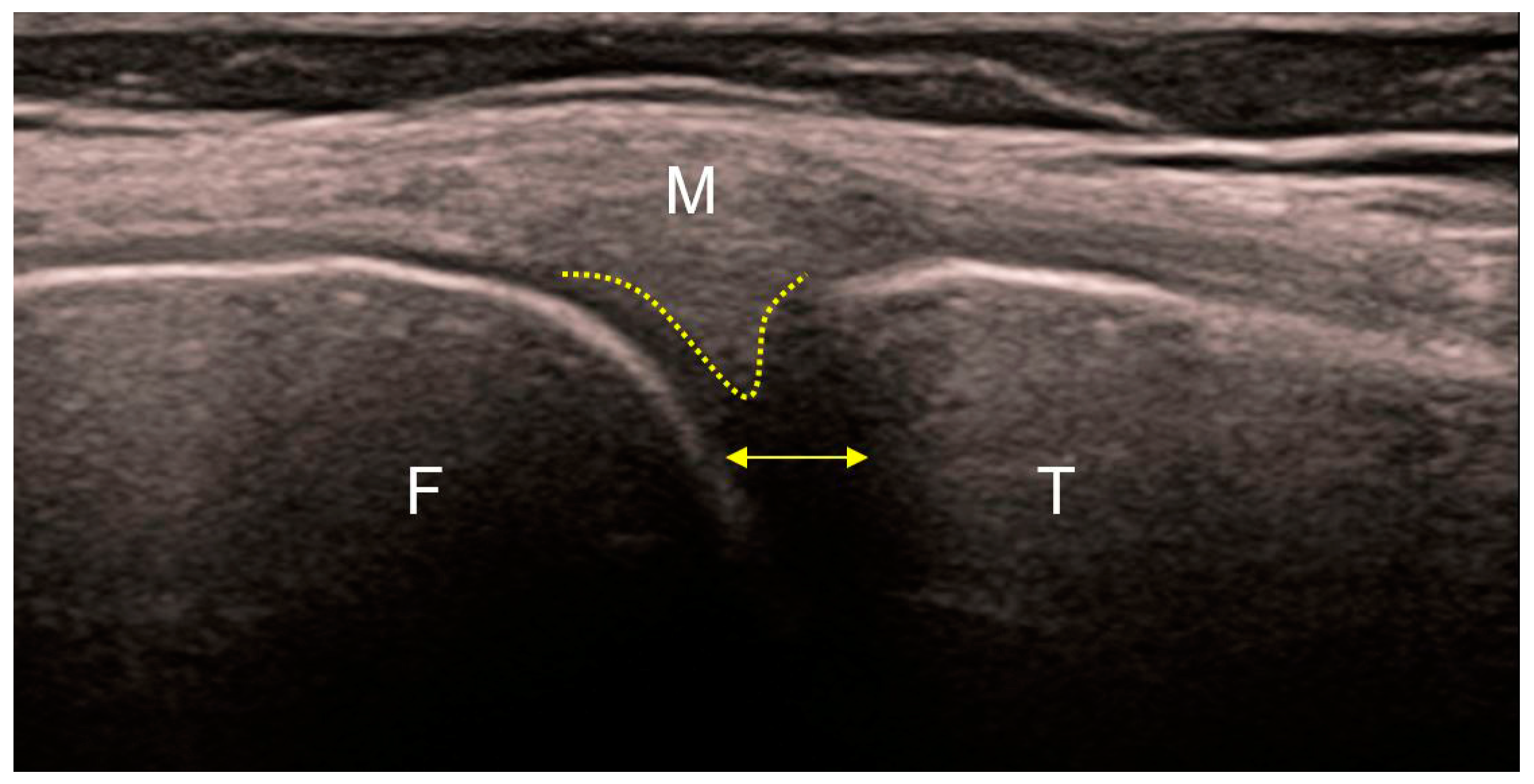
Figure 4. Ultrasound of the medial part of a healthy knee. Regular echoes from the femoral (F) and tibial (T) articular surfaces. The peripheral part of the medial meniscus (yellow dotted line) without a visible boundary continues into the posterior (oblique) fibers of the medial collateral ligament (M) that insert into it. The other (anterior) fibers of the medial collateral ligament continue vertically toward their insertion on the proximal tibia. Normal distance between the articular bodies indicates preserved thickness of the articular cartilages (yellow bidirectional line).
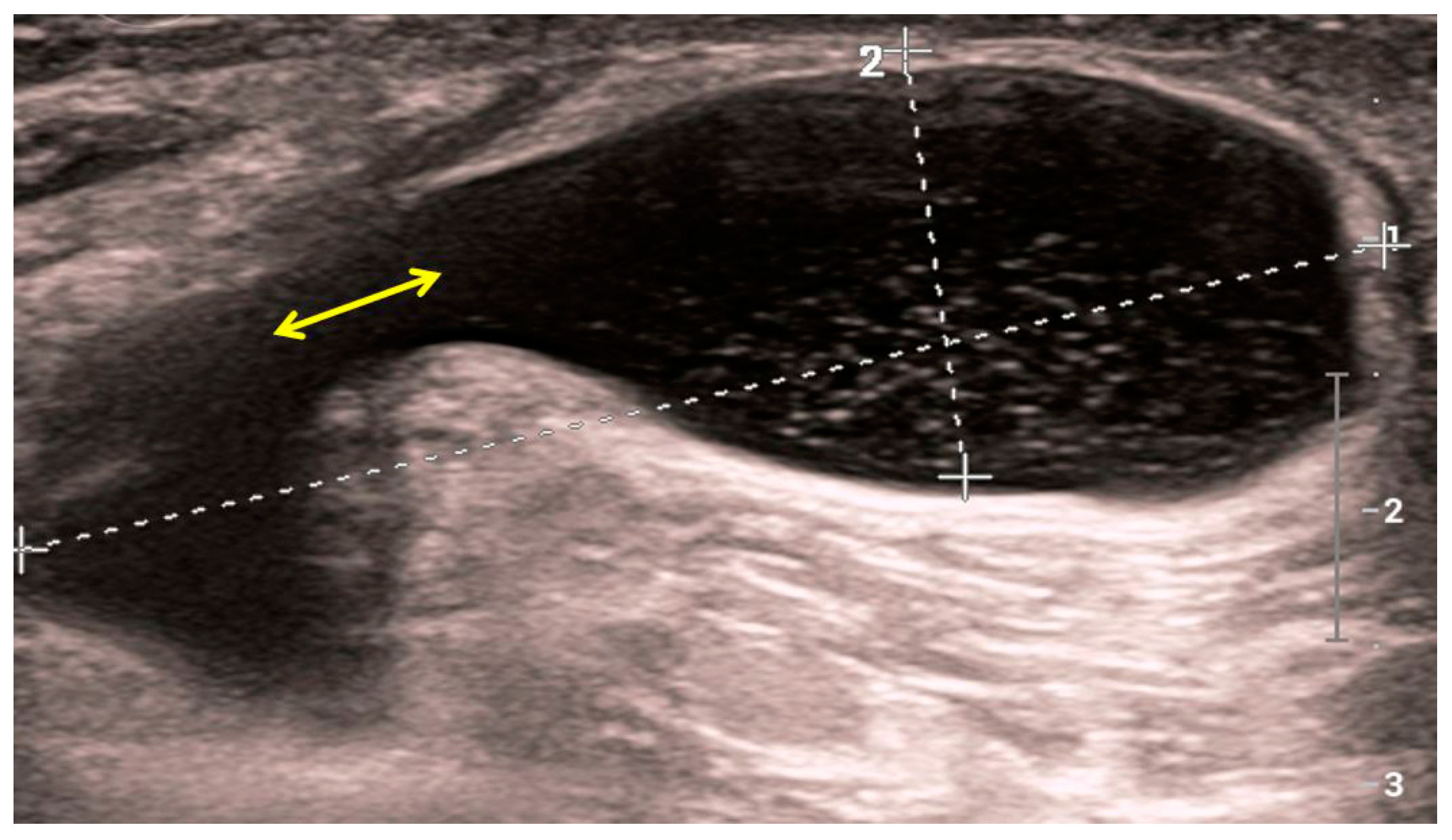
Figure 5. Sonographic image of a Baker’s cyst in the medial part of the popliteal fossa. Punctiform internal echoes from the cyst lumen which communicate with the joint space of the knee joint (yellow bidirectional arrow). Fluid within the cyst enhances the ultrasound beam posteriorly.
Numerous national and international guidelines have been developed for the diagnosis of osteoarthritis, which has been well-received by primary healthcare physicians. According to the British national guidelines from 2022, the diagnosis of osteoarthritis is made in individuals over 45 years of age with joint pain associated with physical activity, if they do not have morning stiffness of the joints or if it does not last longer than 30 min. The routine use of imaging diagnostic methods is not recommended, except in atypical cases that suggest a different diagnosis. This recommendation is supported by the lack of evidence for the contribution of imaging methods in diagnosing osteoarthritis, considering that anamnesis and physical examination of the patient are sufficient for diagnosis, while reducing unnecessary use of healthcare and financial resources [56][20]. A somewhat different point of view is held by a group of international experts, who believe that a positive clinical and typical radiographic finding is sufficient for diagnosing knee osteoarthritis, and further diagnostic imaging with magnetic resonance imaging is not recommended [57][21].
References
- Moskowitz, R.W. Bone remodeling in osteoarthritis: Subchondral and osteophytic responses. Osteoarthr. Cartil. 1999, 7, 323–324.
- Bullough, P. The pathology of osteoarthritis. In Osteoarthritis; Moskowitz, R., Howell, D., Goldberg, V., Mankin, H., Eds.; W.B. Saunders: Philadelphia, PA, USA, 1992; pp. 39–69.
- Matyas, J.R.; Sandell, L.J.; Adams, M.E. Gene expression of type II collagens in chondro-osteophytes in experimental osteoarthritis. Osteoarthr. Cartil. 1997, 5, 99–105.
- Tardif, G.; Pelletier, J.P.; Boileau, C.; Martel-Pelletier, J. The BMP antagonists follistatin and gremlin in normal and early osteoarthritic cartilage: An immunohistochemical study. Osteoarthr. Cartil. 2009, 17, 263–270.
- van Beuningen, H.M.; Glansbeek, H.L.; van der Kraan, P.M.; van den Berg, W.B. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthr. Cartil. 1998, 6, 306–317.
- Langenskiöld, A.; Michelsson, J.E.; Videman, T. Osteoarthritis of the knee in the rabbit produced by immobilization. Attempts to achieve a reproducible model for studies on pathogenesis and therapy. Acta Orthop. Scand. 1979, 50, 1–14.
- Dumic-Cule, I.; Brkljacic, J.; Rogic, D.; Bordukalo Niksic, T.; Tikvica Luetic, A.; Draca, N.; Kufner, V.; Trkulja, V.; Grgurevic, L.; Vukicevic, S. Systemically available bone morphogenetic protein two and seven affect bone metabolism. Int. Orthop. 2014, 38, 1979–1985.
- Dumic-Cule, I.; Peric, M.; Kucko, L.; Grgurevic, L.; Pecina, M.; Vukicevic, S. Bone morphogenetic proteins in fracture repair. Int. Orthop. 2018, 42, 2619–2626.
- van der Kraan, P.M.; van den Berg, W.B. Osteophytes: Relevance and biology. Osteoarthr. Cartil. 2007, 15, 237–244.
- Neuman, P.; Hulth, A.; Lindén, B.; Johnell, O.; Dahlberg, L. The role of osteophytic growth in hip osteoarthritis. Int. Orthop. 2003, 27, 262–266.
- Hanada, K.; Solchaga, L.A.; Caplan, A.I.; Hering, T.M.; Goldberg, V.M.; Yoo, J.U.; Johnstone, B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J. Cell Biochem. 2001, 81, 284–294.
- Uusitalo, H.; Hiltunen, A.; Ahonen, M.; Kahari, V.M.; Aro, H.; Vuorio, E. Induction of periosteal callus formation by bone morphogenetic protein-2 employing adenovirusmediated gene delivery. Matrix Biol. 2001, 20, 123–127.
- Iwasaki, M.; Nakahara, H.; Nakase, T.; Kimura, T.; Takaoka, K.; Caplan, A.I.; Ono, K. Bone morphogenetic protein 2 stimulates osteogenesis but does not affect chondrogenesis in osteochondrogenic differentiation of periosteum-derived cells. J. Bone Miner. Res. 1994, 9, 1195–1204.
- Shirasawa, S.; Sekiya, I.; Sakaguchi, Y.; Yagishita, K.; Ichinose, S.; Muneta, T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: Optimal condition and comparison with bone marrowderived cells. J. Cell Biochem. 2006, 97, 84–97.
- Joyce, M.E.; Roberts, A.B.; Sporn, M.B.; Bolander, M.E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J. Cell Biol. 1990, 110, 2195–2207.
- Anderson-MacKenzie, J.M.; Quasnichka, H.L.; Starr, R.L.; Lewis, E.J.; Billingham, M.E.; Bailey, A.J. Fundamental subchondral bone changes in spontaneous knee osteoarthritis. Int. J. Biochem. Cell Biol. 2005, 37, 224–236.
- Hayami, T.; Pickarski, M.; Zhuo, Y.; Wesolowski, G.A.; Rodan, G.A.; Duong, L.T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 2006, 38, 234–243.
- Xu, L.; Hayashi, D.; Roemer, F.W.; Felson, D.T.; Guermazi, A. Magnetic resonance imaging of subchondral bone marrow lesions in association with osteoarthritis. Semin. Arthritis Rheum. 2012, 42, 105–118.
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502.
- NICE. Osteoarthritis in over 16s: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2022.
- Martel-Pelletier, J.; Maheu, E.; Pelletier, J.P.; Alekseeva, L.; Mkinsi, O.; Branco, J.; Monod, P.; Planta, F.; Reginster, J.Y.; Rannou, F. A new decision tree for diagnosis of osteoarthritis in primary care: International consensus of experts. Aging Clin. Exp. Res. 2019, 31, 19–30.
More
