Alkaline phosphatase is a vital enzyme used in separation studies and as a biomarker for liver, bone, and certain cancer conditions. Its stability and specific properties enable insights into enzyme behavior, aiding in the development of detection methods with broader applications in various scientific fields. Alkaline phosphatase has four main isoenzymes: germ cell alkaline phosphatase (GCAP), intestinal alkaline phosphatase (IAP), placental alkaline phosphatase (PLAP), IAP, PLAP, and tissue-nonspecific alkaline phosphatase (TNAP)TNAP, each with distinct roles. TNAP is found in the liver, kidney, and bones, playing a role in bone mineralization. Separation techniques like electrophoresis and chromatography are valuable for studying enzymes and proteins, revealing insights into their structure and function in pharmaceutical research and post-translational modification (PTM)PTM studies.
- alkaline phosphatase
- capillary electrophoresis
- post-translational modifications
- whole cell analysis
1. Introduction
2. Capillary Electrophoresis (CE)
The history of CE is relatively short compared to other separation techniques, but it has seen rapid advancements and improvements. In 1983, Lukacs and his professor, Professor Jorgenson, published a seminal paper that demonstrated the potential of using capillaries to overcome the limitations of traditional gel electrophoresis methods. They built upon the earlier work of Hjerten and Catsimpoolas, who had developed capillary zone electrophoresis, and identified ways to reduce interferences between bands, thus enhancing the resolution and efficiency of separations [7][8][9][10]. This breakthrough opened the door for further research and development in the field of capillary electrophoresis. Over the years, CE has become a complementary method to other modern separation instruments and has found applications in various scientific fields, including biochemistry, pharmaceuticals, environmental analysis, and more. Capillary electrophoresis (CE) is an advanced form of electrophoresis that uses narrow capillaries filled with a conductive buffer to separate molecules under an electric field. CE offers higher separation efficiency and speed due to the small capillary size and the ability to generate higher electric fields. It requires smaller sample volumes, leading to enhanced sensitivity and lower detection limits. CE systems can be automated and integrated with other techniques, like mass spectrometry [11], for comprehensive enzyme analysis. CE has broad applications in high-throughput analysis, protein characterization, and monitoring enzyme activity in various fields, including pharmaceutical research, clinical diagnostics, and biotechnology. CE can utilize different modes of separation, such as capillary zone electrophoresis (CZE), capillary isoelectric focusing (CIEF), or capillary gel electrophoresis (CGE), depending on the specific application. In CE, the movement of charged molecules through a narrow capillary tube occurs under the influence of an electric field. The electrical double layer forms at the capillary wall, consisting of an inner layer of counterions and an outer layer of co-ions. As analytes migrate through the capillary, they experience electroosmotic flow driven by this double layer, allowing for the separation of molecules based on their charge and size. Figure 1 shows a diagram illustrating the components of CE.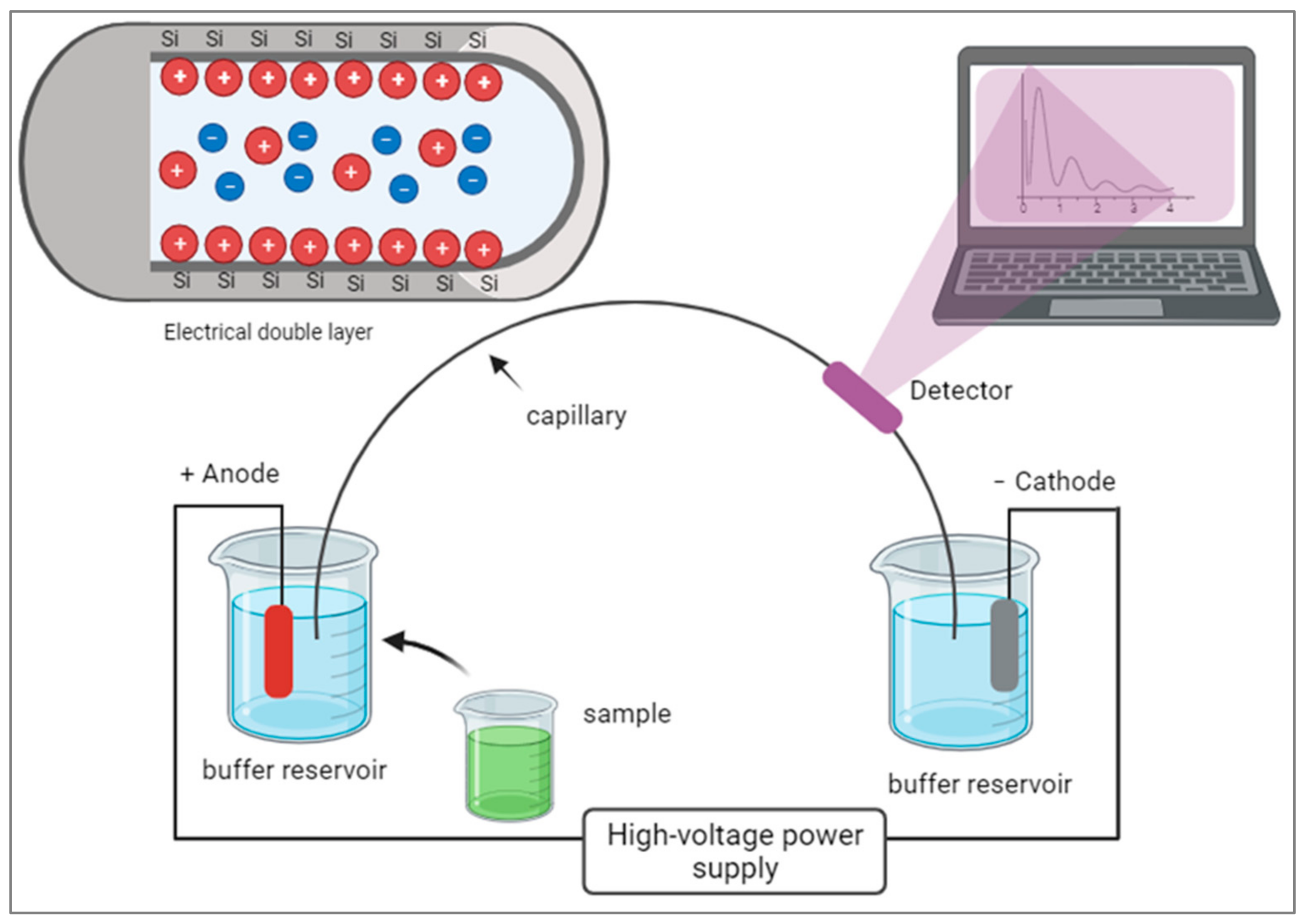
3. Indirect Detection of Alkaline Phosphatase
In the case of a UV-Vis detector, p-nitrophenol (pNP) is the product of the enzyme reaction of alkaline phosphatase and the substrate p-nitrophenol phosphate (pNPP). pNP has chromophores that make it sensitive for spectrometric detection and it has two functional groups: one is the OH and the other is NO2. These occupy opposite ends of the benzene ring. The absorption spectra occur in 405 nm for this compound. By following the steps in Figure 2, capillary electrophoresis with a UV-Vis detector can be used to detect and quantify ALP. Indirect detection is typically performed using discontinuous assays with pre- or post-reaction sampling, as it involves stopping the enzymatic reaction at specific time points to quench the reaction and prevent further product formation. The reaction mixture is then analyzed at each time point to measure the accumulated product or remaining substrate concentration. So, here, the enzyme reaction is initiated by mixing the enzyme with its substrate and other necessary components. After a specific incubation period, the reaction is stopped (e.g., by changing the pH or adding a quenching reagent). A small volume of the reaction mixture is sampled and analyzed via capillary electrophoresis using indirect detection methods. The concentrations of the product(s) or remaining substrate are determined, and the enzyme activity or concentration is calculated based on these measurements. Discontinuous assays provide information about the extent of the enzymatic reaction at specific time points, allowing for the determination of initial reaction rates and steady-state kinetics.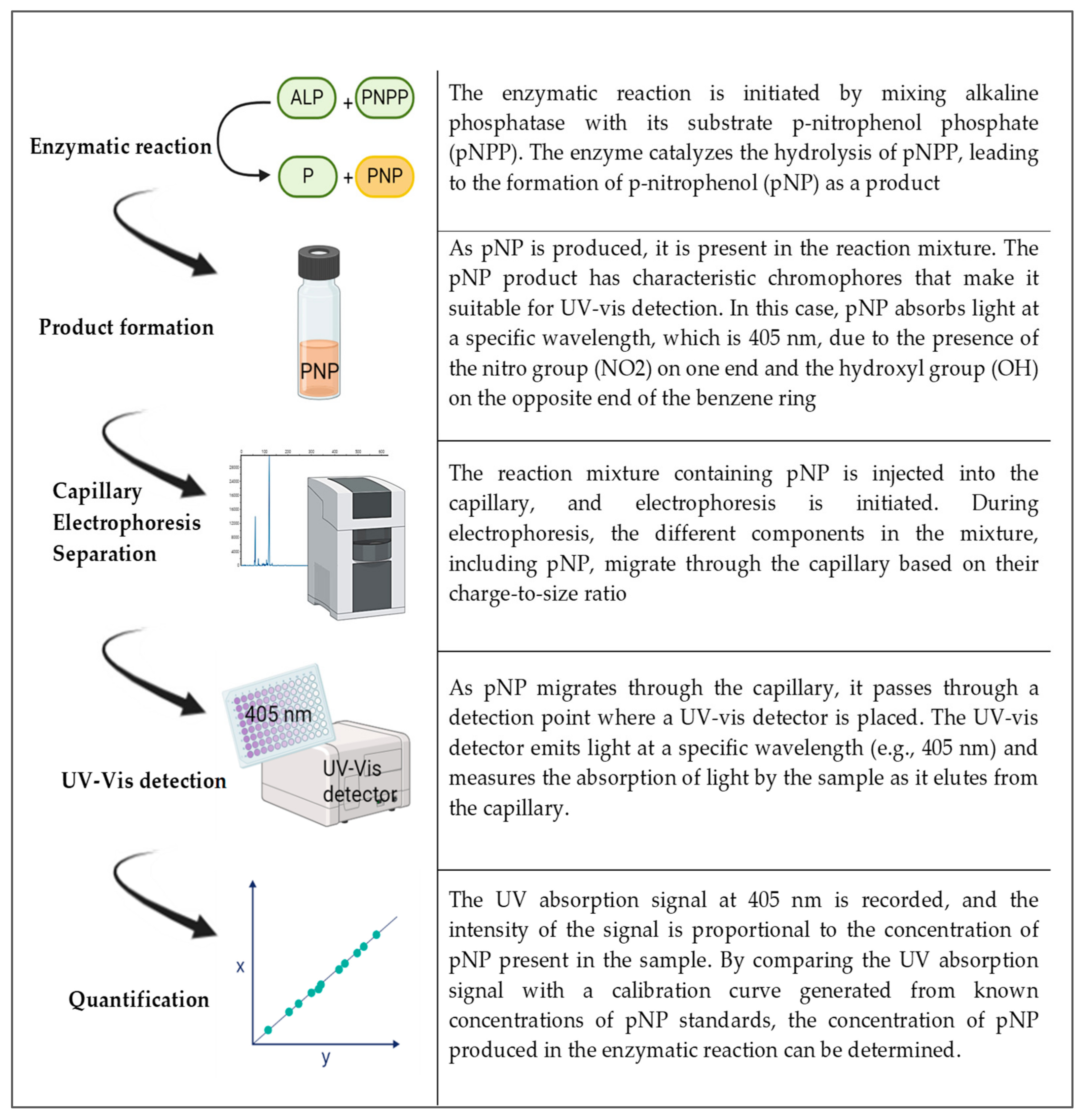
4. On-Column Detection of Alkaline Phosphatase
In this detection method, the substance of interest is detected directly as it moves through the capillary and during its migration it can be real-time-monitored during the enzymatic reaction; therefore, it is called a continuous assay. Figure 3 shows that continuous assays provide kinetic information about the enzymatic reaction and are useful for studying reaction rates, enzyme inhibition, and enzyme kinetics. It involves continuously monitoring the enzymatic reaction as it progresses over time. This approach allows for real-time observation of the reaction kinetics. The enzyme reaction is initiated by mixing the enzyme with its substrate and other necessary components. The reaction mixture is injected into the capillary, and electrophoresis is started. As the reaction progresses, the products of the enzymatic reaction, or any other species involved in the reaction, move through the capillary with different electrophoretic mobilities. These products are detected as they elute from the capillary, and their concentrations are monitored over time, usually through absorbance or fluorescence detection. The rate of product formation or substrate consumption is then used to determine the enzyme’s activity or concentration.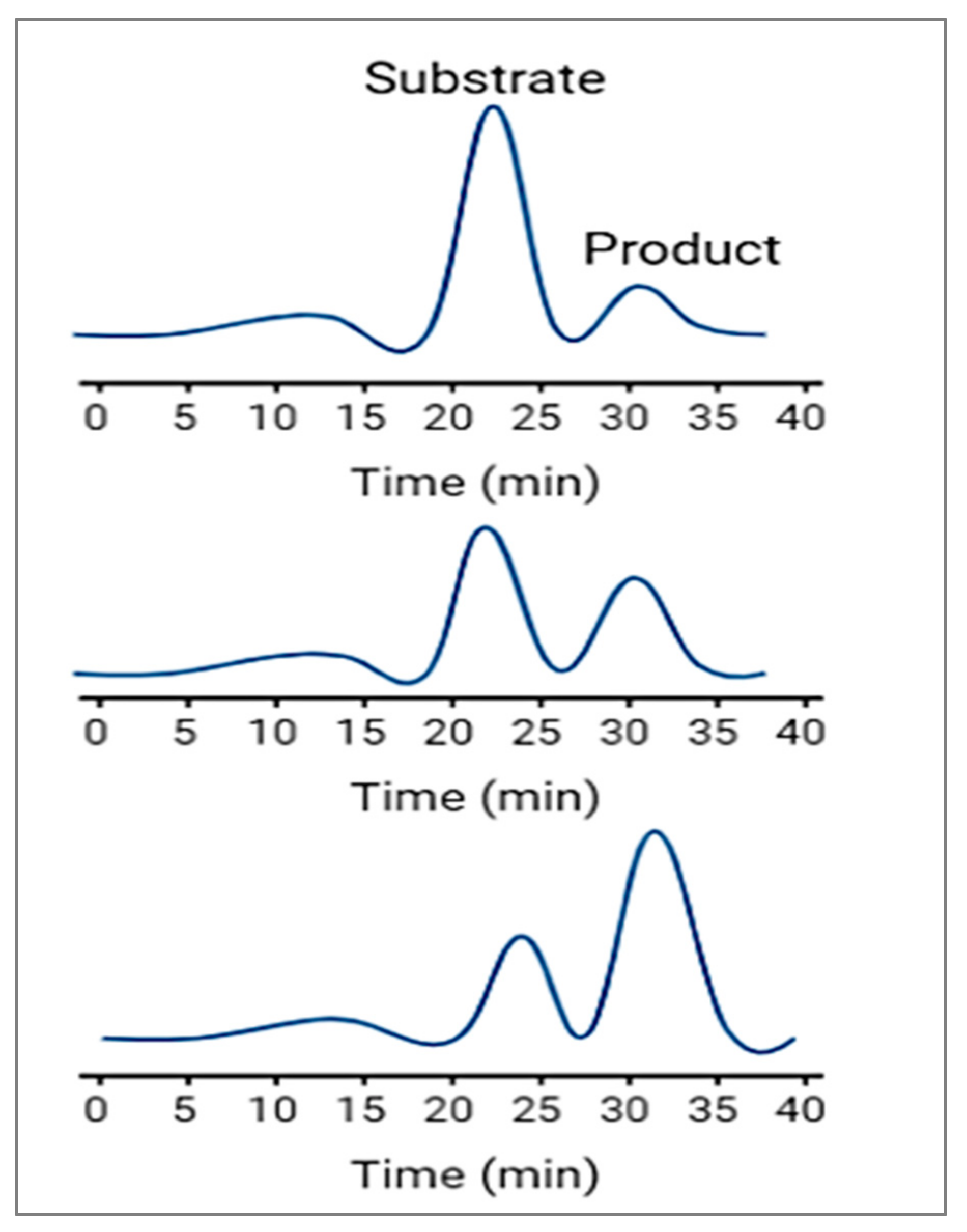
5. Alkaline Phosphatase Detection from Whole Cells
It is important to note that the successful detection of ALP from whole cells using capillary electrophoresis depends on optimizing the sample preparation, separation conditions, and detection methods. Moreover, the sensitivity of the technique should be considered, as the concentration of ALP in whole cells may be relatively low, requiring careful optimization to achieve reliable results. Additionally, the use of complementary techniques like mass spectrometry [28] can provide more comprehensive information about PTMs in complex biological samples. Studying PTMs of ALP using capillary electrophoresis can provide mechanistic insights into how specific modifications regulate the enzyme’s activity, substrate specificity, subcellular localization, and protein–protein interactions. Capillary electrophoresis enables the identification of protein isoforms with distinct post-translational modifications (PTMs) due to their slightly different migration times, allowing for differentiation. Moreover, the intensity of electropherogram peaks in the capillary electrophoresis reflects the protein’s concentration in the sample, enabling researchers to quantify the abundance of specific PTMs and understand the extent of modification. Coupling capillary electrophoresis with mass spectrometry enables comprehensive PTM profiling, facilitating the identification and characterization of specific PTMs on proteins and shedding light on their regulatory roles in various cellular processes. By correlating PTM patterns with specific cellular conditions or disease states, scientists can gain insights into the functional implications of PTMs on protein activity and cellular signaling pathways. Figure 4 shows detecting post-translational modifications (PTMs) in capillary electrophoresis from whole cells, which involves several steps. After releasing cells’ contents in the appropriate buffer, proteins are extracted from the lysate using appropriate solvents. Proteins are then likely to denature and reduce to break down their tertiary and quaternary parts. For targeting PTM-specific enrichment, proteins are modified using different methods, including immunoprecipitation [29] or affinity chromatography [30]. For ALP, PTM-specific detection phosphorylation [29][30][31][32][33] or glycosylation [34][35][36] can be used. After injecting the protein mixture, data can be analyzed and compared to the untreated cell and other controls in the test.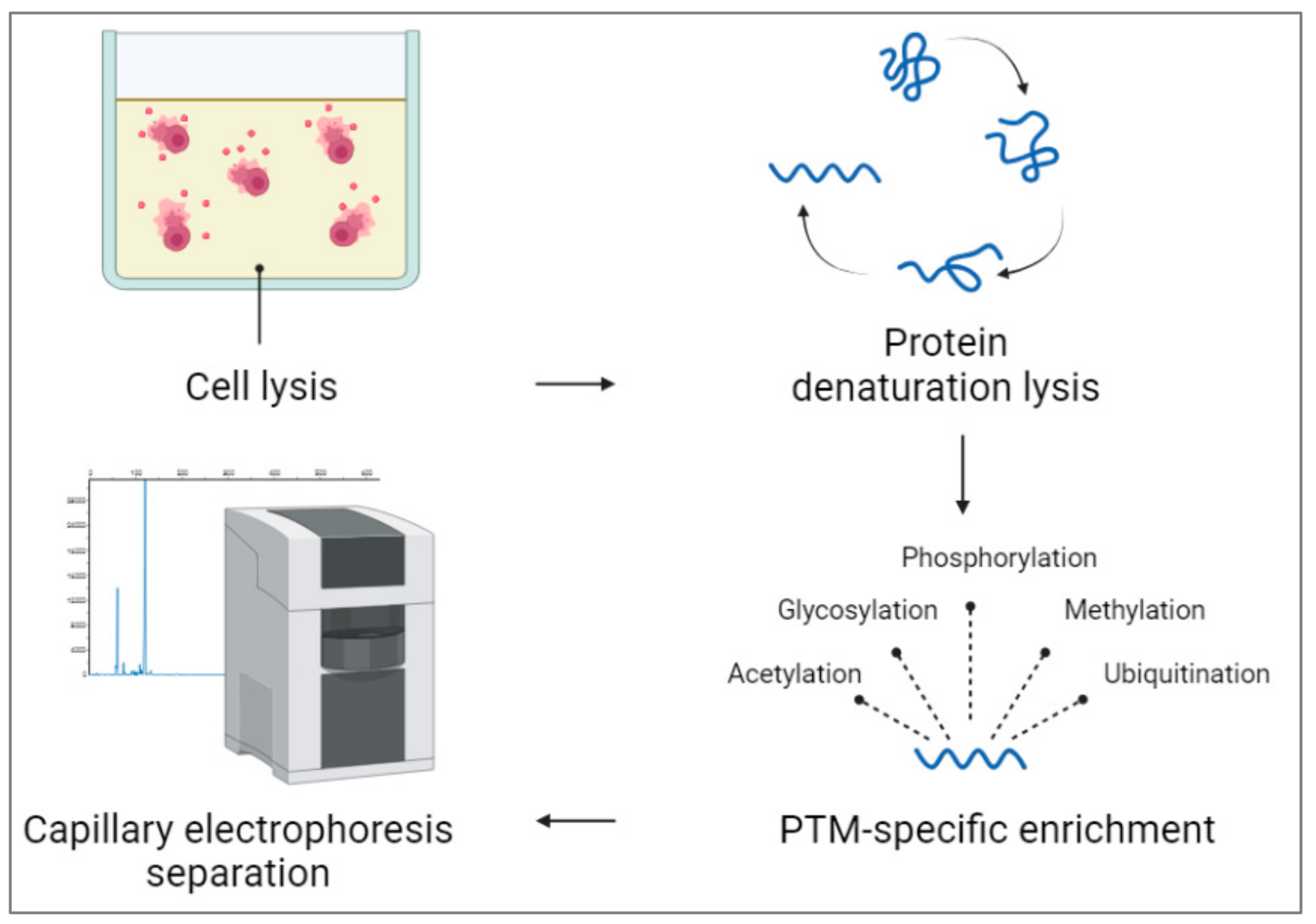
References
- Kwan, Y.H.; Thormann, W. Electrophoretically mediated microanalysis for characterization of the enantioselective CYP3A4 catalyzed N-demethylation of ketamine. Electrophoresis 2012, 33, 3299–3305.
- Řemínek, R.; Glatz, Z.; Thormann, W. Optimized on-line enantioselective capillary electrophoretic method for kinetic and inhibition studies of drug metabolism mediated by cytochrome P450 enzymes. Electrophoresis 2015, 36, 1349–1357.
- McComb, R.B.; Bowers, G.N., Jr.; Posen, S. Alkaline Phosphatase; Plenum Press: New York, NY, USA, 1979.
- Mohamadnia, A.R.; Shahbazkia, H.R.; Sharifi, S.; Shafaei, I. Bone-specific alkaline phosphatase as a good indicator of bone formation in sheepdogs. Comp. Clin. Pathol. 2007, 16, 265–270.
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448.
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278.
- Jorgenson, J.W.; Lukacs, K.D. Zone Electrophoresis in Open-Tubular Glass Capillaries. Anal. Chem. 1981, 53, 1298–1302.
- Jorgenson, J.W.; Lukacs, K.D. Zone electrophoresis in open-tubular glass capillaries: Preliminary data on performance. J. High Resolut. Chromatogr. 1981, 4, 230–231.
- Jorgenson, J.W.; Lukacs, K.D. Capillary zone electrophoresis. Science 1983, 222, 266–272.
- Jorgenson, J.W. Zone electrophoresis in open-tubular capillaries. Trends Anal. Chem. 1984, 3, 51–54.
- McLachlin, D.T.; Chait, B.T. Analysis of phosphorylated proteins and peptides by mass spectrometry. Curr. Opin. Chem. Biol. 2001, 5, 591–602.
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sensors Actuators B Chem. 1990, 1, 244–248.
- Harrison, D.J.; Manz, A.; Lüdi, H.; Widmer, H.M.; Fan, Z. Capillary Electrophoresis and Sample Injection Systems Integrated on a Planar Glass Chip. Anal. Chem. 1992, 64, 1926–1932.
- Manz, A.; Lüdi, H.; Widmer, H.M. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems. Capillary electrophoresis on a chip. J. Chromatogr. A 1992, 593, 253–258.
- Green, J.S.; Jorgenson, J.W. Minimizing adsorption of proteins on fused silica in capillary zone electrophoresis by the addition of alkali metal salts to the buffers. J. Chromatogr. A 1989, 478, 63–70.
- Lee, K.-J.; Heo, G.S. Free solution capillary electrophoresis of proteins using untreated fused-silica capillaries. J. Chromatogr. A 1991, 559, 317–324.
- Bullock, J.A.; Yuan, L.-C. Free solution capillary electrophoresis of basic proteins in uncoated fused silica capillary tubing. J. Microcolumn Sep. 1991, 3, 241–248.
- Gattu, S.; Crihfield, C.L.; Lu, G.; Bwanali, L.; Veltri, L.M.; Holland, L.A. Advances in enzyme substrate analysis with capillary electrophoresis. Methods 2018, 146, 93–106.
- Nguyen, B.T.; Kang, M.J. Application of capillary electrophoresis with laser-induced fluorescence to immunoassays and enzyme assays. Molecules 2019, 24, 1977.
- Craig, D.B.; Wong, J.C.Y.; Dovichi, N.J. Detection of attomolar concentrations of alkaline phosphatase by capillary electrophoresis using laser-induced fluorescence detection. Anal. Chem. 1996, 68, 697–700.
- Whisnant, A.R.; Johnston, S.E.; Gilman, S.D. Capillary electrophoretic analysis of alkaline phosphatase inhibition by theophylline. Electrophor. Int. J. 2000, 21, 1341–1348.
- Murakami, Y.; Morita, T.; Kanekiyo, T.; Tamiya, E. On-chip capillary electrophoresis for alkaline phosphatase testing. Biosens. Bioelectron. 2001, 16, 1009–1014.
- Whisnant, A.R.; Gilman, S.D. Studies of reversible inhibition, irreversible inhibition, and activation of alkaline phosphatase by capillary electrophoresis. Anal. Biochem. 2002, 307, 226–234.
- Neel, C.A. Studies of Alkaline Phosphatase Inhibition by Metal Chelators Using Capillary Electrophoresis. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2005.
- Sarver, S.A.; Keithley, R.B.; Essaka, D.C.; Tanaka, H.; Yoshimura, Y.; Palcic, M.M.; Hindsgaul, O.; Dovichi, N.J. Preparation and electrophoretic separation of Bodipy-Fl-labeled glycosphingolipids. J. Chromatogr. A 2012, 1229, 268–273.
- Li, C.; Wang, H. Selective enzymatic cleavage and labeling for sensitive capillary electrophoresis laserinduced fluorescence analysis of oxidized DNA bases. J. Chromatogr. A 2015, 1406, 324–330.
- Ramana, P.; Adams, E.; Augustijns, P.; Schepdael, A.V. Trapping magnetic nanoparticles for in-line capillary electrophoresis in a liquid based capillary coolant system. Talanta 2017, 164, 148–153.
- DeLaney, K.; Sauer, C.S.; Vu, N.Q.; Li, L. Recent Advances and New Perspectives in Capillary Electrophoresis-Mass Spectrometry for Single Cell ‘Omics’. Molecules 2018, 24, 42.
- Bucci, D.; Isani, G.; Giaretta, E.; Spinaci, M.; Tamanini, C.; Ferlizza, E.; Galeati, G. Alkaline phosphatase in boar sperm function. Andrology 2013, 2, 100–106.
- Kinoshita, E.; Yamada, A.; Takeda, H.; Kinoshita-Kikuta, E.; Koike, T. Novel immobilized zinc(II) affinity chromatography for phosphopeptides and phosphorylated proteins. Anal. Sci. J. 2005, 28, 155–162.
- Kinoshita, E.; Kinoshita-Kikuta, E.; Takiyama, K.; Koike, T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteom. 2006, 5, 749–757.
- Du, M.; Li, X.; Li, Z.; Shen, Q.; Wang, Y.; Li, G.; Zhang, D. Phosphorylation regulated by protein kinase A and alkaline phosphatase play positive roles in μ-calpain activity. Food Chem. 2018, 252, 33–39.
- Gao, R.; Ye, N.; Kou, X.; Shen, Y.; Yang, H.; Wu, T.; Huang, S.; Chen, G.; Ouyang, G. Hierarchically mesoporous CE-based mofs with enhanced alkaline phosphatase-like activity for phosphorylated biomarker sensing. Chem. Commun. 2022, 58, 12720–12723.
- Ouyang, A.; Bennett, P.; Zhang, A.; Yang, S.-T. Affinity chromatographic separation of secreted alkaline phosphatase and glucoamylase using reactive dyes. Process Biochem. 2007, 42, 561–569.
- Linder, C.H.; Narisawa, S.; Millán, J.L.; Magnusson, P. Glycosylation differences contribute to distinct catalytic properties among bone alkaline phosphatase isoforms. Bone 2009, 45, 987–993.
- Olczak, M.; Szulc, B. Modified secreted alkaline phosphatase as an improved reporter protein for N-glycosylation analysis. PLoS ONE 2021, 16, e0251805.
 Encyclopedia
Encyclopedia
