Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Stergios Boussios and Version 2 by Lindsay Dong.
Prostate cancer (PC) is the most common cancer in men and the second leading cause of cancer-related death worldwide. Antiagiogenic therapies have shown substantial benefits for many types of cancer but only a marginal benefit for PC. Despite the important role of angiogenesis in PC, clinical trials in refractory castration-resistant PC (CRPC) have demonstrated increased toxicity with no clinical benefit. A better understanding of the mechanism of angiogenesis may help to understand the failure of trials, possibly leading to the development of new targeted anti-angiogenic therapies in PC. These could include the identification of specific subsets of patients who might benefit from these therapeutic strategies.
- prostate cancer
- castration-resistant prostate cancer
- hormone-sensitive prostate cancer
- antiangiogenics
- advances
1. Introduction
1.1. Pathways Involved in the Angiogenesis of Prostate Cancer
Angiogenesis is a complex multistep process involving endothelial cells, extracellular matrix and soluble factors. It is subdivided into several stages, including proteolytic degradation of the basement membrane and surrounding extracellular matrix, endothelial cell proliferation and migration and finally tube formation and structural reorganization. Prostate cancer (PC) has the ability to produce angiogenic factors of which the most studied are reported in this section. The early angiogenic “initiation switch” correlates expression of hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF) tVEGF tyrosine kinase receptor (VEGFR)-1 in addition to the recruitment and elaboration of intraductal vasculature in prostatic epithelial neoplasia lesions (Figure 1).
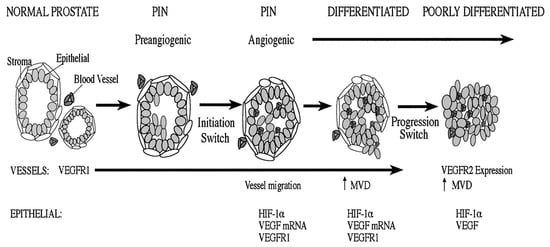
Figure 1. Normal prostate has prominent interductal vasculature, with VEGFR-1 expression. Preangiogenic epithelial neoplasia lesions lesions express VEGFR-1 and demonstrate a hypoxic environment that can stabilize HIF-1α. At this stage, the vasculature is interductal. Concomitant with epithelial neoplasia lesions, there is an angiogenic initiation switch that correlates with noticeable vessel migration into the prostatic duct, and the epithelial cells express HIF-1α. VEGF microRNA (miRNA) is expressed by the tumor cells and VEGFR-1 and protein are expressed by the tumor and endothelial cells. A second-event angiogenic progression switch is consistent with progression to a poorly differentiated tumor. In this environment, endothelial cells express VEGFR-2 and HIF-1α, and a detectable level of VEGF is expressed by tumor cells.
Table 1 depicts the factors that are involved in the angiogenesis in PC.
Table 1.
Factors involved in angiogenesis in prostate cancer.
| Pathway | Factor | Clinical Impact | References |
|---|---|---|---|
| Vascular Endothelial Growth factors (VEGFs) | VEGFR 1, -2 and -3 | Stimulators | [1][2][3][10][36,37][,384][,395][,406][,417][,428][,439,44,45] |
| Fibroblast growth factors (FGFs) | FGF-1 and -2, bFGF | [11][12][13][14][15][16][46,47,48,49,50,51] | |
| Matrix metalloproteinases (MMPs) | MMP-2, -7 and -9 | [17][18][19][20][52,53,54,55] | |
| Transforming growth factor β (TGFβ) | TGFβ1 | [21]56[22][23][24][,5725,58],59[26,60][27][,61,62] | |
| Hypoxia-inducible factors (HIF) | HIF-1a | [28][29][30][31][32][63,64,65,66,67] | |
| Cyclooxygenases (COXs) | COX2 | [33][34][35][36][37][68,69,70,71,72] | |
| Interleukins (ILs) | IL8 | Stimulator | [38][39][40][74,76,77] |
| IL10 and IL27 | Inhibitors | [39][41][42][73,76,78] | |
| microRNAs (miRNAs) | miR-296, miR-30d, miR-323, miR-21, miR-182 and miR-130b | Stimulators | [43][88] |
| miR-195, miR-218 and miR-146a | Inhibitors | [44][87] |
2. Anti-Angiogenic Agents in Prostate Cancer
2.1. VEGF-Directed Agents
3.1. VEGF-Directed Agents
23.1.1. Bevacizumab
Bevacizumab is a humanized monoclonal antibody that recognizes all VEGF isoforms, averting connection to the VEGFR [45][98]. It has been used in combination with chemotherapy for the treatment of locally advanced, recurrent or metastatic non-small cell lung cancer, metastatic colorectal cancer, recurrent/metastatic cervical and ovarian/fallopian tube/peritoneal cancer, as well as for the metastatic breast cancer [only European Medicines Agency (EMA)-approved]. Moreover, it has been approved by the FDA in combination with interferon alfa for the metastatic renal cell cancer and as monotherapy for the recurrent glioblastoma. Historically, single agent bevacizumab was initially tested in 15 patients with CRPC. Bevacizumab was given at a dosage of 10 mg/kg every 2 weeks for 6 cycles [46][99]. There were no patients who had a 50% PSA decline and, therefore, the study was considered negative. It is important to note that antiangiogenic drugs are mainly cytostatic rather than cytotoxic; for this reason, radiographic and PSA are possibly not best approaches to assess clinical response. Interestingly, Iacobelli presented a case report of a patient with castrate-resistant PC who was treated with single agent bevacizumab when he refused chemotherapy [47][100]. Bevacizumab 7.5 mg/kg every 14 days was used for more than 6 months with reduction in prostate-specific antigen (PSA) from 14 to 4 ng/mL in 1 month, as well as radiographic response of retroperitoneal lymphadenopathy.
Bevacizumab has also been evaluated when prescribed in combination with cytotoxic agents in PC. A cooperative group study, CALGB 90006, used bevacizumab in combination with docetaxel and estramustine in PC patients who were chemotherapy-naïve [48][101]. Docetaxel was given at 70 mg/m2 every 3 weeks in combination with estramustine 280 mg three times daily on days one through five plus bevacizumab 15 mg/kg on day 2 of the chemotherapy cycle. The study resulted in a 50% PSA decrease in 77% of patients. Moreover, in a phase II study of 20 patients with docetaxel-refractory mCRPC, bevacizumab was added to standard of care docetaxel [49][102]. Patients were treated with docetaxel 60 mg/m2 and bevacizumab 10 mg/kg every 3 weeks. PSA declines of ≥50% were seen in 55% of patients. A randomized phase III trial compared docetaxel plus prednisone to the merging of docetaxel, prednisone, and bevacizumab in patients who are chemotherapy-naïve [50][103]. Theis study enrolled 1050 patients with chemotherapy-naïve, mCRPC to receive docetaxel plus prednisone (docetaxel 75 mg/m2 on day 1; prednisone 5 mg orally twice daily) with either bevacizumab 15 mg/kg or placebo, given on day 1 of a 21-day cycle. The study failed to achieve its primary endpoint of survival, and the bevacizumab arm showed an excessive percentage of treatment-related toxicity and mortality. The rate of grade 3 adverse events in the bevacizumab arm was 74.8% compared to 55.3% in the placebo arm (p < 0.001). Furthermore, there was a 4.4% toxic death rate on the bevacizumab arm in contrast to a percentage of 1.1% in the placebo arm (p = 0.0014). The greater number of deaths caused due to treatment were infection-related. On the contrary, the bevacizumab arm exhibited superiority in PFS and rates of ≥50% PSA decrease.
23.1.2. Aflibercept
Aflibercept is a fusion protein comprising the extracellular domain of the human VEGFR merged to the Fc part of human immunoglobulin G1 [51][104]. Aflibercept efficiently binds to VEGF-A, VEGF-B and placenta growth factors (PlGF)-1 and -2, and, in this way, it inhibits angiogenesis. It is approved by the FDA for the treatment of patients with metastatic colorectal cancer after failure of an oxaliplatin-based regimen [9][44]. Aflibercept has been examined in phase I and II clinical trials combined with docetaxel; however, major toxicities arose from this combination, such as hypertension, proteinuria, epistaxis, and dysphonia. VENICE is a phase III, randomized, double-blind placebo-controlled trial, involving 1224 chemotherapy-naïve patients with mCRPC, who were randomized to docetaxel, prednisone and aflibercept or to docetaxel, prednisone and placebo [52][105]. The study was negative because of lack of an overall survival (OS)OS advantage in the aflibercept arm. Moreover, a remarkable increase in the percentage of side effects was reported in the aflibercept arm, such as severe gastrointestinal symptoms, hypertension, bleeding, fatigue, infections, and treatment-related fatal adverse events.
2.2. VEGFR Tyrosine Kinase Inhibitors
3.2. VEGFR Tyrosine Kinase Inhibitors
23.2.1. Sorafenib
Multiple tyrosine kinase inhibitors target the VEGFR. Sorafenib is a multi-kinase inhibitor that can target tumor cell proliferation via Raf kinase inhibition and angiogenesis via inhibition of VEGFR-2, VEGFR-3 and PDGF receptor kinases [53][106]. Phase II studies in PC have shown interesting results. Twenty-two patients with metastatic androgen independent PC were enrolled onto a phase II National Cancer Institute (NCI)-sponsored study of sorafenib 400 mg twice daily. The majority of the patients (59%) received more than one line of chemotherapy before enrolling on theis study. Patients with ≥50% PSA decrease were not detected [54][107]. Interestingly, 2 patients with increasing PSA levels exhibited resolution of the lesions in the bone scan, suggesting that sorafenib can stimulate PSA secretion, while exerting the antitumor activity on PC. A phase II European study using sorafenib as a single agent at a dose of 400 mg twice daily has also been performed. The 55 males who took part in the trial all had chemotherapy-naïve CRPC. Among them, 2 patients had ≥50% PSA declines at 12 weeks [55][108]. Sorafenib could possibly have a minor clinical activity in PC as a single agent, although it still needs to be further investigated at earlier stages of the disease, as well as in combination with chemotherapy or other anti-PC treatments.
23.2.2. Sunitinib
Sunitinib is a multi-tyrosine kinase inhibitor that inhibits VEGF and PDGF receptors [56][109]. Michaelson et al. published a phase II study, which included 34 males, half chemotherapy-naïve and half docetaxel-resistant, who were treated with sunitinib 50 mg daily for 4 weeks, followed by 2 weeks rest [57][110]. Among them, only one did not have metastatic disease. Two patients experienced a ≥50% PSA decrease and the best radiographic reaction was partial response in only one patient [57][110].
23.2.3. Cediranib
Cediranib is an oral small molecule inhibitor of VEGFR-1, VEGFR-2 and VEGFR-3 and also of PDGF receptor and c-kit [58][111]. Cediranib has shown more promising results compared to sunitinib and sorafenib when utilized for the treatment of PC. A phase I trial described that up to 20 mg of cediranib can be used with minimal to almost no toxicity [59][112]. A phase II study enrolled 59 patients, 39 of whom had been previously treated with at least 2 chemotherapy combinations. Six out of the 39 patients with measurable disease had partial responses. At 6 months, 43.9% of patients were progression free; the median PFS and OS were 3.7 and 10.1 months, respectively [59][112]. The most common adverse events were fatigue, anorexia, weight loss and hypertension, which were dramatically decreased when prednisolone was added to the regimen. Overall, PSA increase did not correspond to change in imaging, similarly to what observed with sorafenib.
23.2.4. Vandetanib
Vandetanib is an oral multi-tyrosine kinase inhibitor that targets VEGFR-2, EGFR and RET (rearranged during transfection) pathways in cancer [60][113]. Vandetanib was combined with docetaxel/prednisolone and studied in a phase II trial of 86 CRPC patients; no positive results were reported [61][114]. Moreover, in a phase II randomized trial in mCRPC patients, the addition of vandetanib to bicalutamide did not increase the activity of single-agent bicalutamide and was associated with reduced tolerability of the combination [62][115].
23.2.5. Cabozantinib
Cabozantinib is an orally available kinase-inhibitor that targets c-MET, VEGFR, RET and other tyrosine kinases [63][116]. It has shown preclinical activity in a multitude of tumor types, including breast, lung, medullary thyroid, as well as PC and is also considered as a new standard-of-care first and second line treatment option for renal cell carcinoma [64][65][117,118]. Cabozantinib may also provide a new therapeutic choice for patients with radioiodine-refractory differentiated thyroid cancer, who have no available standard of care [66][119]. Preclinical studies have reported that it has activity in PC by preventing angiogenesis, whereas clinical studies have shown promising results in terms of bone lesion resolution, and reduction in patients’ circulating tumor cell (CTC) counts [67][68][120,121]. A phase II randomized trial of cabozantinib was prematurely terminated because of evidence of efficacy in the cabozantinib arm [69][122]. On the contrary, a phase III study of cabozantinib, recruiting patients with previously treated mCRPC, reported no overall improvement in the cabozantinib arm [70][123]. Finally, the combination of the cabozantinib with the immune checkpoint inhibitor atezolizumab achieved encouraging activity in patients with mCRPC, according to the results of COSMIC-021 [71][124]. This is a phase Ib, open-label study with a dose-escalation phase and a multicohort expansion phase. The dose-escalation phase determined that the optimal dose of cabozantinib is 40 mg once daily when used in combination with a 1200 mg infusion of atezolizumab once every 3 weeks. The objective response rate was 32% at a median follow-up of 12.6 months.
2.3. PDGF-Targeted Therapy
3.3. PDGF-Targeted Therapy
Imatinib is a multi-tyrosine kinase inhibitor with anti-PDGF receptor activity; it is approved for the treatment of chronic myelogenous leukemia and gastrointestinal stromal tumors. Lin et al. studied imatinib at a dose of 400 mg orally twice daily in 20 patients with non-metastatic PC and rising PSA [72][125]. Only a single participant had PSA decrease of ≥50% and 11 patients withdrew from the study due to toxic effects. Rao et al. also conducted a phase II study using imatinib 400 mg orally twice daily in 21 patients with biochemical only recurrence; this was an additional trial early terminated, due to unexpectedly rapid PSA rise and moderate toxicity [73][126]. Bajaj et al. also presented the outcome of a study utilizing imatinib 400 mg orally twice daily [74][127]. PSA declines of ≥50% were seen in only 2 out of 27 patients (3.7%). The majority demonstrating PSA relapse (74.1%), whilst grade 3 toxicities were reported in approximately 20% of the participants. Taking the results of the three above mentioned studies into consideration, imatinib 400 mg twice daily seems to have minimal benefit in PC.
The results of other PDGF-targeting have also been examined in patients with metastatic PC. The PDGF receptor inhibitor leflunomide was used in patients with androgen-independent PC in a phase II study of 44 men with metastatic disease. Among them, 22 had already been treated with chemotherapy [75][128]. Leflunomide was given intravenously with a 4-day loading dose. Three patients had PSA decrease of ≥50% and 1 of them experienced a substantial drop from 293 to 0.3 ng/mL. Therefore, there is a chance a minor subgroup of PC to have benefit from PDGF signaling inhibitors.
Lastly, imatinib has been combined with docetaxel in a randomized phase II study, enrolling 144 patients with metastatic androgen-independent PC, who received either imatinib 600 mg daily or placebo combined with 30 mg of intravenous docetaxel on days 1, 8, 15 and 22 of a 42-day cycle [76][129]. The percentage of a PSA decrease of ≥50%, PFS, and OS mostly leaned towards the placebo arm [77][130]. The study was terminated early due to increased toxicity effects, mostly of gastrointestinal origin.
2.4. Antiangiogenic/Immunomodulatory Drugs
3.4. Antiangiogenic/Immunomodulatory Drugs
23.4.1. Thalidomide
Thalidomide has been approved as treatment for various hematological malignancies, including multiple myeloma. However, several studies have reported positive effects in solid tumors as well, including PC [78][131]. Despite its antiangiogenic characteristics not being understood in depth, numerous assays have advocated that the antiangiogenic traits could be secondary to the inhibition of secretion of two angiogenic cytokines, namely, VEGF and FGF from both tumor and stromal cells [79][132]. Thalidomide as a possible treatment for PC was investigated as monotherapy or combined with chemotherapy in mCRPC. A recent phase II study enrolling 63 patients compared low-dose (200 mg/day) and high-dose (up to 1200 mg/day) thalidomide; 27% of the patients experienced a decrease in PSA of ≥40% [80][133]. The evidence of the possible activity of thalidomide as single agent and preclinical demonstration that chemotherapy could magnify the efficacy of antiangiogenic agents constituted the basis for testing the combination of thalidomide and docetaxel in vivo [81][134]. A randomized phase II study enrolled 60 patients with mCRPC to receive intravenous docetaxel and bevacizumab plus oral thalidomide and prednisone. The primary end point was a PSA decrease of ≥50%. Secondary end points included PFS, OS and safety [82][135]. The results showed that 90% of patients receiving the combination therapy had PSA decrease of ≥50%, and 88% achieved a PSA decline of ≥30% within the first 3 months of treatment, while the side effects were manageable.
23.4.2. Lenalidomide
Lenalidomide is a thalidomide derivative that mediates VEGF-mediated PI3K-Akt signaling pathway [83][136]. The outcomes of a phase II clinical study that treated 63 mCRPC patients with a combination of lenalidomide, docetaxel, bevacizumab and prednisone, reported that the therapeutic strategy of combining various angiogenesis inhibitors was safe and could possibly be effective [84][137]. Finally, in a randomized, double-blind, placebo-controlled, phase III study, 1059 chemotherapy-naive mCRPC patients were treated with lenalidomide combined with docetaxel and prednisone. However, they experienced severe adverse reactions, including hematological side effects, diarrhea, pulmonary embolism and asthenia with no apparent therapeutic benefit [85][138].
23.4.3. Miscellaneous Angiogenesis Inhibitors
i. Tasquinimod
-
Tasquinimod
Tasquinimod is an angiogenesis-inhibiting factor that has recently been studied as a potential treatment for PC, based on preclinical results showing activity in human PC xenograft models [86][139]. In a phase I study enrolling patients with mCRPC, tasquinimod showed dose-limiting side effects such as sinus tachycardia and asymptomatic amylase increases [87][140]. A phase II, randomized, double-blind, placebo-controlled trial of 201 patients with chemotherapy-naive mCRPC, demonstrated that oral tasquinimod resulted in a median PFS of 7.6 versus 3.3 months in those receiving placebo (p = 0.004) [88][141]. The most frequent drug-related toxic reactions involved gastrointestinal symptoms, fatigue, musculoskeletal pain and asymptomatic increase of pancreatic enzymes and inflammatory markers.
- ii.
-
ItraconazoleAnother promising drug with anti-angiogenic activity is the antifungal itraconazole [89][142]. It has been examined in a phase II randomized trial, including 46 patients with chemotherapy-naïve mCRPC, receiving either low-dose (200 mg/day) or high-dose (600 mg/day) itraconazole [90][143]. There were no PSA response in the low-dose arm, a PSA decrease was seen in 14% of men in the high-dose arm, and 62% of participants changed to a favorable CTC count (<5 CTCs/7.5 mL blood) after itraconazole treatment. Interestingly, the activity of itraconazole was not consequence of androgen repression, similarly to ketoconazole [91][144]. The major side effects of itraconazole are fatigue, nausea, anorexia and rash, along with a syndrome consisting of hypokalemia, hypertension and edema.
- iii.
-
TrebanabibTrebananib is an innovative peptide-Fc fusion protein that inhibits tumor endothelial cell expansion by inhibiting binding of angiopoietins 1 and 2 and the Tie2 receptor [92][145]. A phase I study included 37 patients and showed evidence of activity in advanced solid tumors [93][146]. Stable disease was observed in 7 patients, 2 of whom experienced stable disease >4 months. The reported adverse events were venous thrombosis, pleural effusion, thrombocytopenia, transient ischemic attack, and cerebral edema with headache and hydrocephalus.Table 2 summarizes the clinical trials of anti-angiogenic therapies, whereas Figure 2 provides the pathways of the angiogenesis and the key antiangiogenic targets in PC.
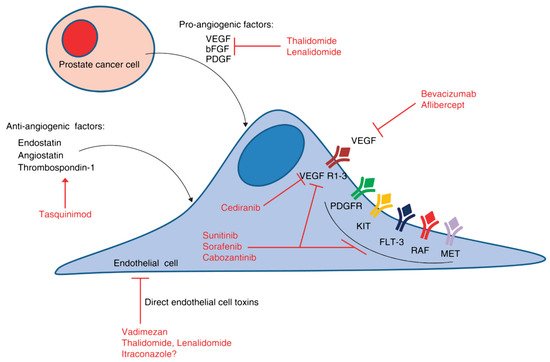 Figure 2. Angiogenic signaling pathways and key antiangiogenic targets in PC. The hypoxic tumor microenvironment stimulates the production of angiogenic cytokines, such as VEGF and basic fibroblast growth factor (bFGF), among others. In addition, there are naturally occurring antiangiogenic factors, such as endostatin and angiostatin, which interact with the proangiogenic factors to determine the overall angiogenic potential of the tumor microenvironment.Table 2. Clinical studies reporting on the activity and safety of anti-angiogenics among patients with prostate cancer.
Figure 2. Angiogenic signaling pathways and key antiangiogenic targets in PC. The hypoxic tumor microenvironment stimulates the production of angiogenic cytokines, such as VEGF and basic fibroblast growth factor (bFGF), among others. In addition, there are naturally occurring antiangiogenic factors, such as endostatin and angiostatin, which interact with the proangiogenic factors to determine the overall angiogenic potential of the tumor microenvironment.Table 2. Clinical studies reporting on the activity and safety of anti-angiogenics among patients with prostate cancer.Agent Mechanism of Action Phase Primary Endpoint Identifier Bevacizumab Recombinant humanized monoclonal
antibody that blocks VEGF-AII ORR NCT01083368 II PSA rFS NCT00776594 Aflibercept Binds to circulating VEGF-A III OS NCT00519285 Sunitinib Receptor tyrosine kinase inhibitor II ≥30% PSA decline NCT00879619 II PFS NCT00734851 Sorafenib II Overall response rate NCT00414388 II ≥50% PSA decline NCT00589420 Cediranib Inhibitor of VEGFR-1, -2, and -3 II PFS NCT00527124 II PFS NCT01260688 Cabozantinib Inhibits VEGFRs, MET, and RET II PFS NCT01428219 Thalidomide Inhibition of VEGF, PI3K/Akt/NF-kappaB, and mTOR pathways II ORR NCT00307294 Lenalidomide Inhibition of VEGF-induced
PI3K/Akt pathway signallingII OS NCT00942578 Tasquinimod S100A9 protein that inhibits VEGF III PFS NCT01234311 Itraconazole Inhibition of the Hedgehog pathway II ≥30% PSA decline NCT01450683 VEGF-A: vascular endothelial growth factor; ORR: objective response rate; PSA rFS: PSA recurrence-free survival; OS: overall survival; PFS: progression free survival; VEGFR: vascular endothelial growth factor receptors; PI3K/Akt NF-kappaB: phosphoinositide 3 kinase/protein kinase B/nuclear factor-kappa B; mTOR: mammalian target of rapamycin.
