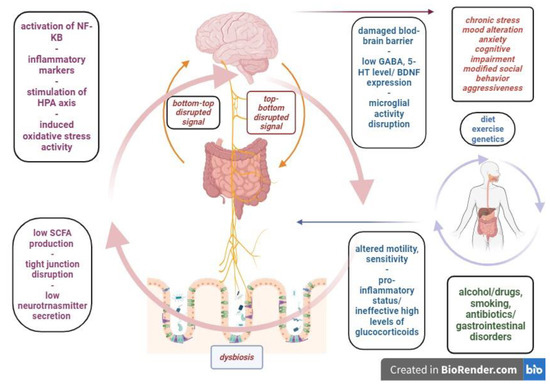
Inflammatory bowel disease represents one of the most life-altering gastrointestinal pathologies, with its multifactorial nature and unclear physiopathology. The most relevant clinical forms, ulcerative colitis and Crohn’s disease, clinically manifest with mild to severe flares and remission periods that alter the patient’s social, familial and professional integration. The chronic inflammatory activity of the intestinal wall determines severe modifications of the local environment, such as dysbiosis, enteric endocrine, nervous and immune system disruptions and intestinal wall permeability changes. These features are part of the gastrointestinal ecosystem that modulates the bottom-to-top signaling to the central nervous system, leading to a neurobiologic imbalance and clinical affective and/or behavioral symptoms. The gut-brain link is a bidirectional pathway and psychological distress can also affect the central nervous system, which will alter the top-to-bottom regulation, leading to possible functional digestive symptoms and local inflammatory responses. In the middle of this neuro-gastrointestinal system, the microbiome is a key player, as its activities offer basic functional support for both relays.
- inflammation
- inflammatory bowel disease
- microbiome
- gut-brain axis
- psychiatry
1. Introduction
2. Functional Considerations over Microbiome and Dysbiosis in Psychiatric Disorders
3. The Gut Barrier and the Blood-Brain Barrier
4. Therapeutic Arguments Linking Dysbiosis, Anxiety and Depression
| Bacteria Species | Digestive Effects | Psychiatric Effects | Source |
|---|---|---|---|
| Lactobacillus reuteri (mice) | Decreased inflammatory activity in colitis | Improvement in anxiety behavior Improvement in depressive symptoms |
[47][22] |
| Lactobacillus mucosae Bifidobacterium longum (mice) |
Synergic effect—inhibition of NF-κB 1, TNF-α 2—anti-inflammatory effect | Anxiety/depression symptom improvement; BDNF 3 expression | [48][23] |
| Lactobacillus plantarum (mice) | Anti-inflammatory effect | BDNF and 5-HT 4 expression Anxiety/depression improvement |
[49][24] |
| Lactobacillus casei (mice) | Anti-inflammatory response, oxidative stress decrease | Mood/behavior/anxiety improvement BDNF expression |
[50][25] |
| Lactococcus lactis subsp. cremoris (mice) | Modulation of inflammatory markers and oxidative stress | Cognitive and mood improvement | [51][26] |
| Bacillus subtilis (human) | Motility, pain improvement Anti-inflammatory effect |
Depression symptom improvement | [52][27] |
| Saccharomyces boulardii (mice) | Anti-inflammatory effect, oxidative stress reduction; gut barrier permeability improvement—histological improvement (LPS 5-induced anxiety) |
5-HT expression Anxiety symptom improvement |
[53][28] |
| Lactiplanbacillus plantarum (mice) | Increased SCFA 6 in induced ulcerative colitis; Gut barrier permeability modulation; Histological improvement; |
5-HT expression; mood improvement; | [54][29] |
| Biotop® (Lactobacillus acidophilus, Clostridium butyricum, Bacillus mesentericus, Streptococcus faecalis) (human) |
Improvement in IBS 7 symptoms of endoscopic remission patients | Social function improvement * L. acidophilus improves depression severity scores in different study with L. casei and B.bifidum |
[55,56][30][31] |
References
- Jiménez-Fernández, S.; Gurpegui, M.; Díaz-Atienza, F.; Pérez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative Stress and Antioxidant Parameters in Patients with Major Depressive Disorder Compared to Healthy Controls Before and after Antidepressant Treatment: Results from a Meta-Analysis. J. Clin. Psychiatry 2015, 76, 13705.
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. 2017, 46, 77–89.
- Bustos-Fernández, L.M.; Hanna-Jairala, I.; Bustos-Fernández, L.M.; Hanna-Jairala, I. Eje Cerebro Intestino Microbiota. Importancia Práctica Clínica. Rev. Gastroenterol. Perú 2022, 42, 106–116.
- Sonali, S.; Ray, B.; Ahmed Tousif, H.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells 2022, 11, 1362.
- Liu, Y.; Xu, F.; Liu, S.; Liu, G.; Yang, X.; Gao, W.; Fan, K.; Zhao, H.; Ma, J. Significance of Gastrointestinal Tract in the Therapeutic Mechanisms of Exercise in Depression: Synchronism between Brain and Intestine through GBA. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 103, 109971.
- Zhu, G.; Zhao, J.; Wang, G.; Chen, W. Bifidobacterium Breve HNXY26M4 Attenuates Cognitive Deficits and Neuroinflammation by Regulating the Gut-Brain Axis in APP/PS1 Mice. J. Agric. Food Chem. 2023, 71, 4646–4655.
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519–1528.
- Ge, X.; Zheng, M.; Hu, M.; Fang, X.; Geng, D.; Liu, S.; Wang, L.; Zhang, J.; Guan, L.; Zheng, P.; et al. Butyrate Ameliorates Quinolinic Acid-Induced Cognitive Decline in Obesity Models. J. Clin. Investig. 2023, 133, e154612.
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve β-Cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542.
- Huang, W.; Guo, H.-L.; Deng, X.; Zhu, T.-T.; Xiong, J.-F.; Xu, Y.-H.; Xu, Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105.
- Lee, S.H. Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases. Intest. Res. 2015, 13, 11–18.
- Santilli, A.; Stefanopoulos, S.; Cresci, G.A.M. The Gut Barrier and Chronic Diseases. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 178–185.
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105.
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut Reactions: How the Blood-Brain Barrier Connects the Microbiome and the Brain. Exp. Biol. Med. 2018, 243, 159–165.
- Wu, H.; Wang, J.; Teng, T.; Yin, B.; He, Y.; Jiang, Y.; Liu, X.; Yu, Y.; Li, X.; Zhou, X. Biomarkers of Intestinal Permeability and Blood-Brain Barrier Permeability in Adolescents with Major Depressive Disorder. J. Affect. Disord. 2023, 323, 659–666.
- Fowlie, G.; Cohen, N.; Ming, X. The Perturbance of Microbiome and Gut-Brain Axis in Autism Spectrum Disorders. Int. J. Mol. Sci. 2018, 19, 2251.
- Varanoske, A.N.; McClung, H.L.; Sepowitz, J.J.; Halagarda, C.J.; Farina, E.K.; Berryman, C.E.; Lieberman, H.R.; McClung, J.P.; Pasiakos, S.M.; Philip Karl, J. Stress and the Gut-Brain Axis: Cognitive Performance, Mood State, and Biomarkers of Blood-Brain Barrier and Intestinal Permeability Following Severe Physical and Psychological Stress. Brain Behav. Immun. 2022, 101, 383–393.
- Geng, S.; Yang, L.; Cheng, F.; Zhang, Z.; Li, J.; Liu, W.; Li, Y.; Chen, Y.; Bao, Y.; Chen, L.; et al. Gut Microbiota Are Associated with Psychological Stress-Induced Defections in Intestinal and Blood-Brain Barriers. Front. Microbiol. 2020, 10, 3067.
- Hao, W.-Z.; Li, X.-J.; Zhang, P.-W.; Chen, J.-X. A Review of Antibiotics, Depression, and the Gut Microbiome. Psychiatry Res. 2020, 284, 112691.
- Uzbay, T. Germ-Free Animal Experiments in the Gut Microbiota Studies. Curr. Opin. Pharmacol. 2019, 49, 6–10.
- Minayo, M.d.S.; Miranda, I.; Telhado, R.S. A Systematic Review of the Effects of Probiotics on Depression and Anxiety: An Alternative Therapy? Ciênc. Saúde Coletiva 2021, 26, 4087–4099.
- Jang, H.-M.; Lee, K.-E.; Kim, D.-H. The Preventive and Curative Effects of Lactobacillus Reuteri NK33 and Bifidobacterium Adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819.
- Kim, S.-K.H. and D.H. Lactobacillus Mucosae and Bifidobacterium Longum Synergistically Alleviate Immobilization Stress-Induced Anxiety/Depression in Mice by Suppressing Gut Dysbiosis. J. Microbiol. Biotechnol. 2019, 29, 1369–1374.
- Sun, X.; Zhang, H.-F.; Ma, C.-L.; Wei, H.; Li, B.-M.; Luo, J. Alleviation of Anxiety/Depressive-Like Behaviors and Improvement of Cognitive Functions by Lactobacillus Plantarum WLPL04 in Chronically Stressed Mice. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, e6613903.
- Yang, Y.; Zhao, S.; Yang, X.; Li, W.; Si, J.; Yang, X. The Antidepressant Potential of Lactobacillus Casei in the Postpartum Depression Rat Model Mediated by the Microbiota-Gut-Brain Axis. Neurosci. Lett. 2022, 774, 136474.
- Ramalho, J.B.; Spiazzi, C.C.; Bicca, D.F.; Rodrigues, J.F.; Sehn, C.P.; da Silva, W.P.; Cibin, F.W.S. Beneficial Effects of Lactococcus Lactis Subsp. Cremoris LL95 Treatment in an LPS-Induced Depression-like Model in Mice. Behav. Brain Res. 2022, 426, 113847.
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus Coagulans MTCC 5856 for the Management of Major Depression with Irritable Bowel Syndrome: A Randomised, Double-Blind, Placebo Controlled, Multi-Centre, Pilot Clinical Study. Food Nutr. Res. 2018, 62, 1218.
- Babaei, F.; Mirzababaei, M.; Mohammadi, G.; Dargahi, L.; Nassiri-Asl, M. Saccharomyces Boulardii Attenuates Lipopolysaccha-ride-Induced Anxiety-like Behaviors in Rats. Neurosci. Lett. 2022, 778, 136600.
- Huang, Y.; Wu, Y.; Jia, X.; Lin, J.; Xiao, L.; Liu, D.; Liang, M. Lactiplantibacillus Plantarum DMDL 9010 Alleviates Dextran Sodium Sulfate (DSS)-Induced Colitis and Behavioral Disorders by Facilitating Microbiota-Gut-Brain Axis Balance. Food Funct. 2022, 13, 411–424.
- Lee, J.; Park, S.B.; Kim, H.W.; Lee, H.S.; Jee, S.R.; Lee, J.H.; Kim, T.O. Clinical Efficacy of Probiotic Therapy on Bowel-Related Symptoms in Patients with Ulcerative Colitis during Endoscopic Remission: An Observational Study. Gastroenterol. Res. Pract. 2022, 2022, e9872230.
- Rayyan, Y.M.; Agraib, L.M.; Alkhatib, B.; Yamani, M.I.; Abu-Sneineh, A.T.; Tayyem, R.F. Does Probiotic Supplementation Improve Quality of Life in Mild-to-Moderately Active Ulcerative Colitis Patients in Jordan? A Secondary Outcome of the Randomized, Double-Blind, Placebo-Controlled Study. Eur. J. Nutr. 2023, 62, 3069–3077.
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillza-deh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Dou-ble-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320.
- El Dib, R.; Periyasamy, A.G.; de Barros, J.L.; França, C.G.; Senefonte, F.L.; Vesentini, G.; Alves, M.G.O.; Rodrigues, J.V.d.S.; Gomaa, H.; Gomes Júnior, J.R.; et al. Probiotics for the Treatment of Depression and Anxiety: A Systematic Review and Meta-Analysis of Random-ized Controlled Trials. Clin. Nutr. ESPEN 2021, 45, 75–90.
- Bambling, M.; Edwards, S.C.; Hall, S.; Vitetta, L. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: An intestinal anti-inflammatory response is suggested. Inflammopharmacology 2017, 25, 271–274.
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and Immune System: From the Mediterranean Diet to Dietary Supplementary through the Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090.
- Järbrink-Sehgal, E.; Andreasson, A. The Gut Microbiota and Mental Health in Adults. Curr. Opin. Neurobiol. 2020, 62, 102–114.
- Zeng, C.; Qiu, Y.; Li, S.; Teng, Z.; Xiang, H.; Chen, J.; Wu, X.; Cao, T.; Zhang, S.; Chen, Q.; et al. Effect of Probiotic Supplements on Oxidative Stress Biomarkers in First-Episode Bipolar Disorder Patients: A Randomized, Placebo-Controlled Trial. Front. Pharmacol. 2022, 13, 829815.
