Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Sayed Amer.
In DNA typing or genetic fingerprinting, variable elements are isolated and identified within the base pair sequences that form the DNA. The person’s probable identity can be determined by analysing nucleotide sequences in particular regions of DNA unique to everyone.
- DNA fingerprinting
- genetic fingerprinting
- autosomal trait screening
1. Introduction
In 1985, Jeffreys discovered DNA profiling, which is one of the most important and prominent developments in the security and criminal fields [1,2,3][1][2][3]. DNA profiling, also known as genetic fingerprinting (GF) or DNA typing, is the process by which a person expresses only one copy of a gene (either from the mother or the father) and suppresses the other copy. GF was used to identify people by comparing specific segments of DNA. It was also used to address parentage issues and filiation disputes. Several other fields, such as the medical, environmental, and agricultural sectors, also benefitted from the application of GF [1,2,3][1][2][3]. According to Jeffreys, who documented the initial development of multilocus DNA fingerprinting, these individual-specific DNA patterns could provide a powerful approach to personal identification and paternity testing. At the time, it was anticipated that these applications would take a while to develop and that significant legal problems would arise when DNA evidence moved from the research lab to the courtroom [1,2][1][2]. In April 1985, the first case involving a dispute over immigration to the UK was successfully resolved using DNA fingerprinting [1]. Shortly afterwards, a UK civil court accepted DNA evidence in a paternity case. The Enderby murder case in October 1986 marked the debut of DNA typing in criminal investigations. This investigation led to the first case of the release of a prime suspect who was later proved innocent by DNA evidence [1]. As early as 1987, DNA typing results were admitted as evidence in criminal courts in the UK and the USA. In 1988, the UK Home Office and Foreign and Commonwealth Office approved the use of DNA fingerprinting to resolve immigration disputes involving disputed family relationships [1,3,4][1][3][4]. GF has developed and evolved by passing through several technical stages until it recently settled to employ a group of genetic sites, as these sites are characterised by high individual strength, are spread on all the chromosomes that make up the human genome and contain short-length tandem passages. Therefore, these sites are of great importance when examining decomposing forensic samples. The technique employed is called short tandem repeats (STRs). STR is the optimum technology used in examining and analysing genetic traits in fingerprinting laboratories, as it suits working conditions in the criminal and forensic fields [5,6][5][6].
Among the genetic sites, microsatellites (MS) are 1-6 base pairs of tandem repeats found within introns and are subject to insertion/deletion events [7]. They are polymorphic loci, as the number of their repeat units varies from one individual to another. When the variation in their number occurs in or near a gene, a change in the function of that gene could be produced. MS have acquired an important oncological interest as they represent the main sign of instability, which is recorded by the expansion or contraction of their sequences in tumour DNA as compared to the normal DNA from the same individual [8]. When multiple unstable genomic loci are found, the tumour is characterized by high-frequency microsatellite instability (MSI), while those displaying only one unstable genomic locus are referred to as low-frequency MSI. Microsatellite stability is considered when none of the analysed loci exhibits instability [9]. Microsatellite instability (MSI) is a molecular fingerprint arising because of defective mismatch repair genes (MLH1, MSH2, PMS1, PMS2, MSH6, or MSH3). Such defects can be inherited (i.e., germline insertion/deletion in the MLH1 gene) or sporadic (e.g., inactivation of MLH1 through hypermethylation of its promoter) [10]. MSI shows the accumulation of mutations in microsatellite DNA repeat sequences spread throughout the genome. It is considered a major biomarker for familial cancer risk assessment, cancer prognosis, and therapeutic choices. Mismatch repair genes are an evolutionarily conserved system preserving DNA homeostasis [11] by recognizing and repairing nucleotide mispairing or insertion/deletion generated during DNA replication, recombination, or damage [12]. Genetic and epigenetic inactivation of mismatch repair genes cause their defects, inducing genome cancer [13].
2. GF Technique
The characteristics of all living organisms, including humans, are essentially determined by the information contained in the DNA they have inherited from their parents. The molecular structure of DNA can be thought of as a two-strand zipper, with nucleotides being the teeth of that zipper. Each tooth is represented by one of the four letters (A, C, G or T), and the opposing teeth form one of two pairs, either A-T or G-C. The letters A, C, G and T stand for adenine, cytosine, guanine and thymine, the basic building blocks of DNA. The information contained in DNA is primarily determined by the sequence of letters along the zipper. For example, the sequence AAT stands for different information than the sequence TAA, although they use the same letters. The process of GF was developed in 1985 by the geneticist Alec Jeffreys. He discovered that some sections of strands of DNA contained sequences of nucleotides repeated next to each other. He also discovered that these sequences are found in the same order of nitrogenous bases in all humans but differ from person to person regarding the number of times they are repeated [1]. They are fixed in their arrangement but differ in the number of repetitions. They were employed to differentiate among humans and determine individuals’ identities. Since these repeated sequences are inherited and transmitted from parents to children, they can also be employed in the resolution of questions of paternity [1,2][1][2]. A repeated sequence of nitrogenous bases on a DNA strand is categorised according to its length—that is, the number of nitrogenous base pairs in the sequence—into one of three types (long, medium-length and short repetitive sequences), all of which are called satellites. Long repetitive sequences, or macrosatellites, contain hundreds to thousands of pairs of nitrogenous bases and are found in certain regions on the chromosomes of the human genome, specifically in the heterochromatic areas near the centromere and at the ends of telomeres; they are also found on the male Y chromosome. Medium-length repetitive sequences, also known as minisatellites, contain between 10 and 100 pairs of nitrogenous bases. They include the repetitive sequences found at a variable number of tandem repeat (VNTR) loci, which are present in some parts of the euchromatic regions on various chromosomes in the human genome. Short repetitive sequences, or microsatellites, contain 1 to 6 pairs of nitrogenous bases [7]. They include the repetitive sequences found at short tandem repeat (STR) loci, which are located in many parts of the euchromatic regions on all chromosomes in the human genome [16,17,18][14][15][16]. GF has evolved in two distinct stages. The first stage relied on the use of VNTR analysis to detect repeating sequences of medium length (minisatellites; 10 to 100 base pairs) and the subsequent use of the restriction fragment length polymorphism (RFLP) technique to analyse the genetic traits of the DNA in the genetic loci. The VNTR technique for GF analysis was exclusively used for approximately 10 years after its discovery [19][17]. In the mid-1990s, after the discovery of short repetitive sequences (microsatellites), the second stage of GF—STR analysis—emerged. This technique, also called simple sequence repeat (SSR) genotyping, examines the genetic traits of DNA through the analysis of genetic sites in which these short sequences are repeated. STR analysis is the latest GF technique, and it is currently the optimal technique for screening for genetic traits at DNA sites. This technique is easy and quick, and the analysis is performed in an automated manner; it is easy to amplify DNA segments using the polymerase chain reaction (PCR) technique because the repetitive sequences are very short (2 to 7 base pairs). Furthermore, this technique allows for clear and accurate results to be obtained on the same day, and the fact that these genetic sites are large and scattered on all chromosomes in the human genome, in addition to the presence of a high percentage of heterozygote genetic sites (approximately 70%), means that its power to differentiate between individuals is very high. Moreover, the STR technique is characterised by its ability to analyse several genetic sites simultaneously; it can also be used to analyse partial or very small quantities of repeats [19,20][17][18]. STR analysis is the preferred technique for the development and collection of latent print evidence in GF and forensic laboratories worldwide [21][19]. Genetic loci that contain short repetitive sequences are known as STR loci, STR markers or genomic fingerprinting loci. The human genome contains many STR markers scattered on all somatic and sex chromosomes, where they occur at a rate of 1 per 10,000 nitrogenous bases. The sites usually spread in the non-coding regions, and they are located either (1) outside the boundaries of genes, in the regions that separate genes on the DNA strand or (2) within the boundaries of genes, in what are called introns [22][20]. Genes consist of two parts: exons and introns. Exons, which are the functional parts of each gene in which the process of gene expression occurs, produce the proteins responsible for genetic traits. Introns are the non-functional parts of genes in which no gene expression occurs and no type of protein is produced. Consequently, GF sites are named according to their locations. A genetic site located within a particular gene is designated by the gene’s name, while the genetic sites that lie outside the boundaries of known genes are named based on the chromosomal positions of the loci in the human genome. Each genetic locus on autosomal chromosomes contains two variants of the gene (called alleles) because there are two copies of autosomes in each cell (one inherited from each parent). The allele at a DNA site is the number of repeats of the sequence of nucleotides at that site. If the alleles are different, they appear as two different numbers, such as the genotype ‘9,7’; in this case, the genotype is heterozygous. If the alleles are identical (i.e., the same number of repetitions—for example, ‘8,8’), the genotype is homozygous. As for the fingerprinting sites on sex chromosomes, each genetic locus contains only one allele because each cell contains one copy of the sex chromosomes; a female carries the X allele at the locus on her chromosome, while a male carries the Y allele at the locus on his chromosome. The main 13 STR loci used for DNA fingerprinting analysis—the Combined DNA Index System (CODIS) core and extra STR loci—are located on the autosomal chromosomes of the human genome [23[21][22],24], in addition to the amelogenin on the sex chromosomes (Figure 1).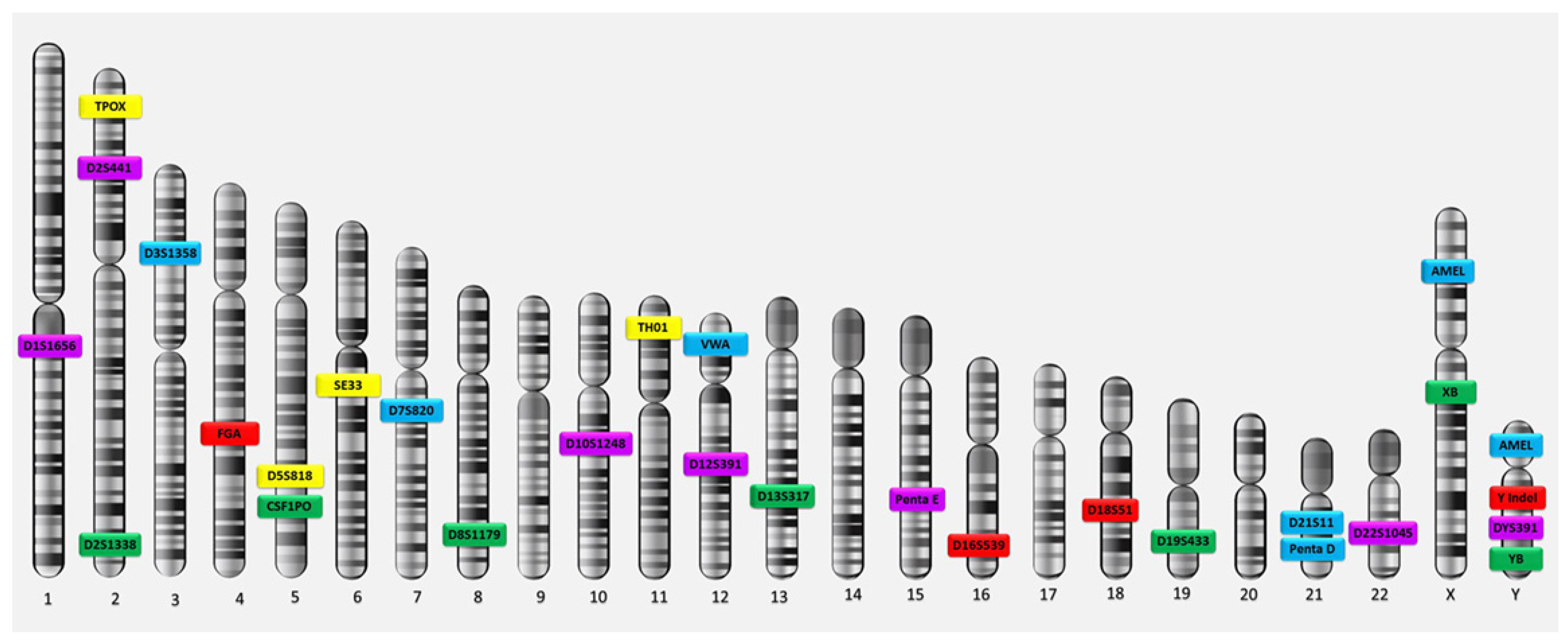
Figure 1. CODIS Core (TPOX, D3S1358, FGA, D5S818, CSF1PO, D7S820, D8S1179, TH01, VWA, D13S317, D16S539, D18S51, D21S11) and extra forensic STR loci on human chromosomes.
3. Medical Applications of GF in Carcinomas
A DNA fingerprint is the same for every cell, tissue, and organ in a person, unlike a traditional fingerprint, which is only found on the fingertips and can be altered by surgery. As a result, DNA fingerprinting is rapidly replacing other methods to recognise and distinguish unique people. An additional application of GF technology is the diagnosis of disorders and diseases in humans. Detecting disorders and diseases early allows the medical team to provide effective treatment. GF has been used in many medical applications, arguably the most important of which is the detection of cancerous tumours [26][23]. Cancer is a large group of more than 100 complex or composite diseases. In most cases, doctors usually perform a biopsy to confirm the presence of cancer. In a biopsy, a sample of the abnormal tissue is taken. A pathologist examines the tissue under a microscope and performs further tests on the cells of the sample. Although an elevated level of a circulating tumour marker can occasionally be helpful in diagnosing cancer and can indicate the presence of cancer, it is not sufficient on its own to make the diagnosis. Thus, it happens that the levels of certain tumour markers rise due to non-cancerous conditions [27][24]. These diseases differ in their behaviours according to the types of cells from which they originated, but all types of cancer share two characteristics: (1) abnormalities in the processes of cell division and growth and (2) the ability of cells to spread (metastasise) and invade tissues other than those from which they originated. The main reason for the emergence of cancer is damage to DNA, specifically in the coding regions on chromosomes, which contain genes and are where gene expression occurs [28][25]. During this process, a defect or error occurs during DNA repair, which leads to a change in the sequence; when the DNA is doubled and cells divide, fixed genetic mutations occur because of that defect. Then, incorrect codes are copied and translated by messenger RNA, resulting in abnormal proteins; these may affect the functions of some genes (e.g., activating oncogenes or inhibiting tumour-suppressor genes that work to control and regulate cell growth). Therefore, cell growth and division continue abnormally, and apoptosis (programmed cell death) does not occur. When DNA damage occurs, signals are released during the cessation of division (resting period) to control the p53 gene in the next stage, after which the cell cycle stops, allowing DNA repair; alternatively, the cell will die by apoptosis if the damage is not repaired. In some cells, the p53 gene may not be active or stimulated because of cellular damage. This may lead to the failure of the cell cycle to stop at the control point, causing the cell to continue for the rest of its life cycle with errors or damage to the DNA without repair or apoptosis occurring. In this case, the cell accumulates the types of mutations or chromosomal changes that lead to cancer development [29][26]. Apoptosis is one of the vital processes necessary to maintain the positions of cells in tissues as well as their balance. During this programmed cell death process, a cell kills itself during growth via the shrinkage and decomposition of the contents of the nucleus and the disintegration of DNA into very small pieces, and the remains of the dead cell are consumed by the healthy neighbouring cells. Apoptosis can be inhibited by tumour-suppressing genes because of exposure to genetic mutations. This allows cells with damaged DNA to grow abnormally and become cancerous [30][27]. MS regions, or STR markers, are essential tools for mapping disease-causing genes in relation to both forensic investigations and population genetics studies (Figure 2).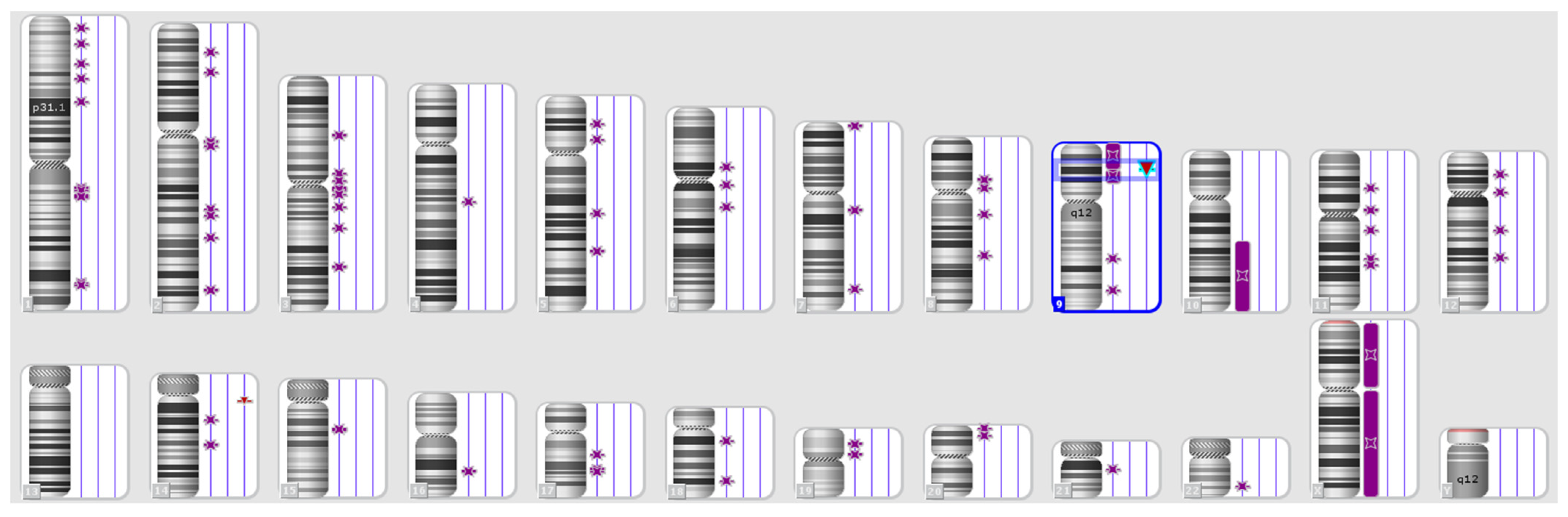
Figure 2. Karyotype of a patient with the loss of heterozygosity and normal karyotype according to the standard cytogenetic analysis. Purple symbols refer to loss of heterozygosity.
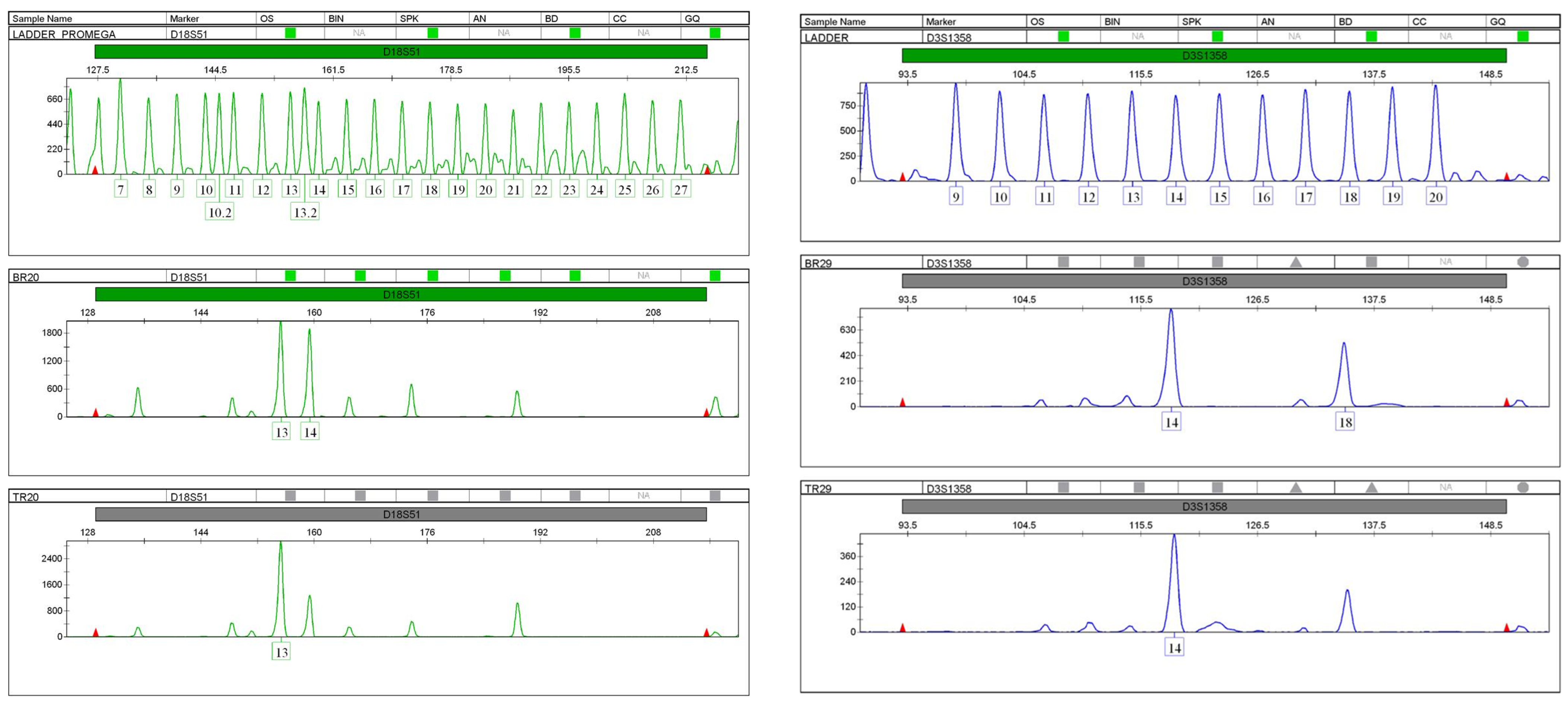
Figure 3. Loss of heterozygosity in STR profiles (D18S51 and D3S1358) of the patient’s breast tumour compared to the STR profile of her normal tissue.
References
- Jeffreys, A.J.; Pena, S.D.J. Brief introduction to human DNA fingerprinting. In DNA Fingerprinting: State of the Science; Birkhauser Verlag Bas: Basel, Switzerland, 1993; pp. 1–20.
- Roewer, L. DNA fingerprinting in forensics: Past, present, future. Investig. Genet. 2013, 4, 22.
- Jordan, D.; Mills, D. Past, present, and future of DNA typing for analyzing human and non-human forensic samples. Front. Ecol. Evol. 2021, 9, 646130.
- Fang, J.; Zhu, X.; Wang, C.; Shangguan, L. Applications of DNA technologies in agriculture. Curr. Genom. 2016, 17, 379–386.
- McDermott, A.M.; Baidouri, H.; Woodward, A.M.; Kam, W.R.; Liu, Y.; Chen, X.; Ziemanski, J.F.; Vistisen, K.; Hazlett, L.D.; Nichols, K.K.; et al. Short tandem repeat (STR) profiles of commonly used human ocular surface cell lines. Curr. Eye Res. 2018, 43, 1097–1101.
- Stanley, U.N.; Khadija, A.M.; Bukola, A.T.; Precious, I.O.; Davidson, E.A. Forensic DNA profiling: Autosomal short tandem repeat as a prominent marker in crime investigation. Malays. J. Med. Sci. 2020, 27, 22–35.
- Toth, G.; Gaspari, Z.; Jurka, J. Microsatellites in different eukaryotic genomes: Survey and analysis. Genome Res. 2000, 10, 967–981.
- Schmidt, M.H.; Pearson, C.E. Disease associated repeat instability and mismatch repair. DNA Repair. 2016, 38, 117–126.
- Palmieri, G.; Casula, M.; Manca, A.; Palomba, G.; Sini, M.C.; Doneddu, V.; Cossu, A.; Colombino, M. Genetic instability markers in cancer. Biomark. Immunother. Cancer Methods Mol. Biol. 2020, 396, 133–154.
- Palmieri, G.; Colombino, M.; Cossu, A.; Marchetti, A.; Botti, G.; Ascierto, P.A. Genetic instability and increased mutational load: Which diagnostic tool best direct patients with cancer to immunotherapy? J. Transl. Med. 2017, 15, 17.
- Gilson, P.; Merlin, J.L.; Harlé, A. Detection of microsatellite instability: State of the art and future applications in circulating tumour DNA (ctDNA). Cancers 2021, 13, 1491.
- Kunkel, T.A. Evolving views of DNA replication (in) fidelity. Cold Spring Harb. Symp. Quant. Biol. 2009, 74, 91–101.
- Li, Z.; Pearlman, A.H.; Hsieh, P. DNA mismatch repair and the DNA damage response. DNA Repair. 2016, 38, 94–101.
- Thakur, J.; Packiaraj, J.; Henikoff, S. Sequence, chromatin and evolution of satellite DNA. Int. J. Mol. Sci. 2021, 22, 4309.
- Audano, P.A.; Sulovari, A.; Graves-Lindsay, T.A.; Cantsilieris, S.; Sorensen, M.; Welch, A.E.; Dougherty, M.L.; Nelson, B.J.; Shah, A.; Dutcher, S.K.; et al. Characterizing the major structural variant alleles of the human genome. Cell 2019, 176, 663–675.
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A.; et al. The complete sequence of a human genome. Science 2022, 376, 44–53.
- Hernandez, Y. A Study of the Variability of Minisatellite Tandem Repeat Loci in the Human Genome Based on High-Throughput Sequencing Data. Ph.D. Thesis, Boston University, Boston, MA, USA, 2019.
- Elkins, K.M.; Berry, H.E.; Reese, K.R. Applications of NGS in DNA Analysis; Springer Singapore EBooks; Springer Nature: Singapore, 2022.
- Menchhoff, S.I.; Solomon, A.D.; Cox, J.O.; Hytinen, M.E.; Miller, M.T.; Cruz, T.D. Effects of storage time on DNA profiling success from archived latent fingerprint samples using an optimised workflow. Forensic Sci. Res. 2022, 7, 61–68.
- Fan, H.; Chu, J.Y. A brief review of short tandem repeat mutation. Genom. Proteom. Bioinform. 2007, 5, 7–14.
- Butler, J.M. The future of forensic DNA analysis. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140252.
- Wyner, N.; Barash, M.; McNevin, D. Forensic autosomal short tandem repeats and their potential association with phenotype. Front. Genet. 2020, 11, 884.
- Samuelsson, J.K.; Alonso, S.; Yamamoto, F.; Perucho, M. DNA fingerprinting techniques for the analysis of genetic and epigenetic alterations in colorectal cancer. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2010, 693, 61–76.
- Tarin, D. Understanding Cancer: The Molecular Mechanisms, Biology, Pathology and Clinical Implications of Malignant Neoplasia; Springer Nature: San Diego, CA, USA, 2023.
- Hull, R.; Francies, F.Z.; Oyomno, M.; Dlamini, Z. Colorectal cancer genetics, incidence and risk factors: In search for targeted therapies. Cancer Manag. Res. 2020, 12, 9869–9882.
- Kaur, R.P.; Vasudeva, K.; Kumar, R.; Munshi, A. Role of p53 gene in breast cancer: Focus on mutation spectrum and therapeutic strategies. Curr. Pharm. Des. 2018, 24, 3566–3575.
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87.
- Nojadeh, J.N.; Sharif, S.B.; Sakhinia, E. Microsatellite instability in colorectal cancer. Exp. Clin. Sci. J. 2018, 17, 159–168.
- Chen, M.L.; Chen, J.Y.; Hu, J.; Chen, Q.; Yu, L.X.; Liu, B.R.; Qian, X.P.; Yang, M. Comparison of microsatellite status detection methods in colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 1431–1438.
More
