Acute ischemic stroke (AIS) is the loss of neurological function due to a sudden reduction in cerebral blood flow and is a leading cause of disability and death worldwide. The field of radiological imaging has experienced growth in recent years, which could be boosted by the advent of artificial intelligence. One of the latest innovations in artificial intelligence is radiomics, which is based on the fact that a large amount of quantitative data can be extracted from radiological images, from which patterns can be identified and associated with specific pathologies. Since its inception, radiomics has been particularly associated with the field of oncology and has shown promising results in a wide range of clinical situations. The performance of radiomics in non-tumour pathologies has been increasingly explored in recent years, and the results continue to be promising.
1. Introduction
Acute ischemic stroke (AIS) is the loss of neurological function due to a sudden reduction in cerebral blood flow and is a leading cause of disability and death worldwide
[1]. According to the WHO, stroke is the second leading cause of disability and death worldwide after ischemic heart disease, accounting for 11% of all deaths. With improvements in the quality of life and longevity of patients, the incidence of stroke is expected to continue to rise in the coming years
[2]. More than 80% of strokes are ischemic in origin
[3]. Recent advances in the management of these patients have focused more on their treatment, where endovascular therapies have radically changed the prognosis of these patients
[4][5][6][4,5,6].
Radiomics is a radiological discipline based on the idea that images are more than just pictures and that there is a lot of data that is invisible to the human eye, but which we can quantify and analyze by looking for data patterns associated with certain pathologies. Initially, radiomics was mainly focused on the field of oncology
[7][9]. All cancer patients have at least one staging CT scan, so the most commonly used imaging technique for feature extraction in these studies was the CT
[8][10]. The aims of these studies have been diverse, ranging from identifying prognostic factors or estimating overall survival to predicting response to treatment. The use of radiomics for gene determination (also called radiogenomics) has also been developed in pathologies such as brain tumours
[9][11], lung cancer
[10][12] and abdominal tumours
[11][13].
In the field of neuroradiology, radiomics features obtained from magnetic resonance imaging (MRI) were used most frequently in the articles. In these articles, radiomics also focused mainly on oncological patients. The objectives ranged from differentiation of high-grade gliomas from brain metastases, prediction of EGFR mutations, or prognostic prediction, to determination of overall survival. In recent years, the use of radiomics has extended to brain pathologies other than tumours. There are articles on the use of radiomic features for prognostic prediction in acute ischemic stroke (AIS)
[12][14], for predicting degenerative diseases
[13][15], or even for predicting aneurysm rupture
[14][16].
2. Radiomics
2.1. Radiomics Workflow
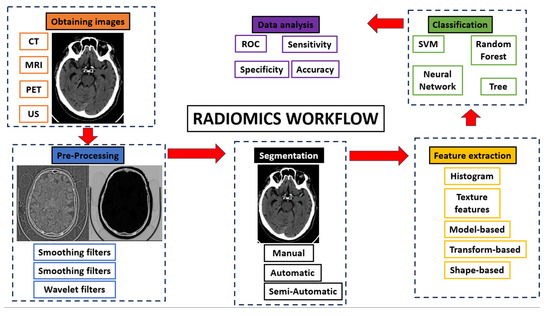
The radiomics workflow consists of five consecutive steps (
Figure 1):
Figure 1.
Radiomics workflow.
-
Obtaining images: Radiomics can be applied to all types of medical imaging. There are published articles on the performance of radiomics in ultrasound, Non-Contrast Enhancement-CT (NCECT), Computed Tomography Angiography (CTA), MRI and PET. In this step, there can be a great deal of heterogeneity when using images acquired in different hospitals or even on different machines within the same hospital.
-
Pre-processing: The quality of the images can be improved by using the pre-processing tools. In this step, some image filters are used to reduce noise. The aim is to increase the predictive power of the classifiers
[15][17].
-
Segmentation: In this step, the region of interest is selected in the radiological image. The segmentation of this region can be performed in three ways: manual, semi-automatic and automatic. Manual segmentation is the gold standard and the most commonly used method in the studies. The main advantage of this model is the intervention of an expert radiologist in its performance. The main disadvantage of this model is the time required to manually segment the entire region of interest
[16][18]. Automatic segmentation is based on automatic detection of the region of interest without human intervention. Finally, semi-automatic segmentation is performed under the supervision of an expert radiologist who can edit an initial automatic pre-segmentation. The advantage of this method is the speed of the segmentation and the fact that the human component remains
[17][19]. With today’s increasingly sophisticated segmentation software, semi-automatic 3D segmentation of an area of interest can be performed quickly and comfortably for the radiologist.
-
Feature extraction and classification: There is a wealth of numerical data that can be extracted from medical images, known as radiomic features. There are several classes of radiomic features: Histogram features (grey level mean, maximum, minimum, variance and percentiles), texture features (absolute gradient, grey level co-occurrence matrix—GLCM—, grey level run length matrix—GLRLM—, grey level size zone matrix—GLSZM—, grey level distance zone matrix—GLDZM—, Neighborhood Grey Level Difference Matrix—NGTDM—, and Neighborhood Grey Level Dependence Matrix—NGLDM—), model-based features, transform-based features (Fourier, Garbor and Wavelet) and shape-based features (geometric properties of ROIs)
[18][20]. These numerical data are classified by automatic classifiers. These classifiers are capable of recognizing different groups of patterns depending on the objective we set for them.
-
Data analysis.
2.2. Radiomics in AIS
Articles analyzing the performance of a radiomic model in neurological pathology have become increasingly frequent. The most frequently published articles are about neurological tumour pathology. However, in recent years there has been an increase in the publication of articles about non-tumour neurological pathology.
Regarding the application of radiomics in acute cerebral ischemic pathology, there has been an exponential increase in the publication of articles since 2018. The objectives of these articles on radiomics in acute ischemic stroke are the prognostic prediction of AIS patients, the detection of early ischemic stroke, prediction of the outcome of revascularization treatment, prediction of complications, prediction of the etiology of AIS, prediction of the time since stroke and differentiation between hemorrhage or contrast extravasation in patients undergoing mechanical thrombectomy.
2.3. Prognostic Prediction
The use of radiomics for the prognostic prediction of AIS patients is the most commonly published. Avery et al. in their study of 829 patients used radiomic features (RFs) obtained from CTA images of patients with anterior circulation Large Vessel Occlusion (LVO). They used masks of the middle cerebral artery (MCA) territory to extract the RF. They used four different input sets: radiomics (only RFs of MCA territory), radiomics + treatment (RF, mTICI score and intravenous thrombolysis), clinical + treatment (clinical variables and treatment) and combined (RF, treatment and clinical variables). The clinical variables included were sex, age and admission NIHSS. The best results were very similar between the clinical + treatment model and the combined model. The area under the curve (AUC) for long-term outcome prediction was 0.81 and 0.82 for the clinical + treatment and combined models, respectively. The AUC for predicting discharge outcome was 0.82 and 0.81 for the clinical + treatment and combined models, respectively.
2.4. Detection of Ischemic Stroke
In the detection of ischemic stroke, articles focus on the early detection of ischemic lesions and the prediction of recurrence. The group of Guan Y et al. showed in their study with 56 patients and 1301 RFs that there is a correlation between RFs and AIS detection (best Acc of 0.7748)
[19][36]. Guo Y et al. also investigated the detection of ischemic stroke (IS) and the prediction of NIHSS and other outcomes using RFs obtained from DSC-PWI in 88 patients. The best AUC they obtained was 0.925 for stroke detection. For the prediction of NIHSS and other prognostic predictions, they obtained an AUC of 0.853 and 0.828, respectively
[20][37].
On the other hand, Zhang R et al. used MRI-based radiomics to predict the ischemic penumbra in their study of 241 patients. They extracted 896 radiomic features from the DWI high signal area using manual segmentation. The AUC obtained was 0.92, and sensitivity, specificity and accuracy were 0.93, 0.75 and 0.82, respectively, in the training cohort. In the validation cohort the AUC, sensitivity, specificity and accuracy were 0.90, 0.88, 0.74 and 0.80, respectively.
In ischemic stroke recurrence prediction, Su et al. developed a combined model with some clinical factors (age, sex, number of chronic ischemic lesions, routine laboratory tests, carotid ultrasound, cardiovascular risk factors and medical treatment) and CT-based radiomics to predict future ischemic stroke in patients with silent lacunar infarction. They obtained a C-index of 0.786 and 0.714 in the training and validation cohorts, respectively. They concluded that radiomic features can provide important information for predicting future ischemic stroke in patients with silent lacunar infarction
[21][40].
2.5. Treatment Predictions
Regarding radiomic models predicting the outcome of reperfusion treatments, most studies focus on predicting the first-pass effect or the TICI scale after thrombectomy. Only one study focuses on predicting recanalization after alteplase treatment.
To predict first-pass recanalization, Hofmeister et al. performed clot segmentation in their study of 156 patients. They used RFs to identify patients with first-pass recanalization by aspiration. They also predicted the total number of passes required to achieve good recanalization with a mechanical thrombectomy device (MTB). They achieved an AUC of 0.88 for predicting patients with first attempt recanalization. They concluded that clot-based radiomics can predict an MTB strategy in patients with AIS
[22][43].
In recanalization prediction, Zhang et al. attempted to predict successful recanalization (TICI ≥ 2c) using MRI-based radiomics in 141 AIS patients. They extracted 321 radiomic features from the ischemic lesion and performed a manual segmentation. They obtained an AUC of 0.7442, a sensitivity of 0.7556 and a specificity of 0.7675. They concluded that MRI-based radiomics may be useful in predicting patient response to thrombectomy
[23][46].
In terms of predicting recanalization after treatment with alteplase, Qiu et al. obtained an AUC of 0.85. They concluded that clot-based radiomics was more predictive of recanalization with alteplase than other classic thrombus imaging features (length, volume and permeability)
[24][49].
The performance of radiomic models in predicting the outcome of thrombectomy in patients with AIS is very good. In this situation, radiomics can provide valuable information for the immediate management of these patients, since knowing in advance the risk of achieving inadequate reperfusion with a specific thrombectomy technique may lead the neuroradiologist to change the recanalization strategy in the event of an unsuccessful first pass.
2.6. Prediction of Complications after AIS
There are some studies that have used RFs to predict some complications in patients with AIS. Fu B et al. obtained an AUC of 0.96 in predicting malignant cerebral edema (MCE) using a combined model with RFs from NECT of patients with AIS
[25][50]. Jiang et al. also predicted edema after acute ischemic stroke using MRI-based radiomics in 389 patients, with similar results
[26][51].
On the other hand, Meng et al. attempted to predict hemorrhage transformation in AIS patients based on MRI radiomics features. They extracted 5400 radiomic features from 71 AIS patients. They performed manual segmentation. They obtained an AUC of 911 and an accuracy of 0.894 in predicting hemorrhage transformation with a combined model. They concluded that a combined model with MRI-based radiomics can provide important information to physicians in the diagnosis of AIS patients
[27][53].
2.7. Etiology Prediction
In the etiology prediction of AIS using radiomics models, Chen Y et al. tried to differentiate between cardioembolic and atherosclerotic etiology in patients with acute ischemic stroke. They included 82 patients, and they obtained RFs from CTA images. They performed a manual segmentation of the embolic region and extracted 116 RFs. In their results, the best AUC was 0.9018 and the best Acc was 0.8929. They concluded that radiomics can effectively predict the subtype of ischemic stroke and help doctors to correctly manage these patients
[28][56].
2.8. Time since Stroke Prediction
Chen Y et al. attempted to estimate the time since stroke onset (TSS). They manually outlined the clot on CTA images of 221 patients and obtained 944 RFs. They divided the patients into two different groups: <4.5 h and >4.5 h since stroke onset. They analyzed the performance of the radiomics model and the combined model (radiomics + clinical data). The AUC was 0.803 for the combined model and 0.813–0.803 for the combined model (training and test cohorts). The clinical data used were age, diabetes and atrial fibrillation. They concluded that there was no significant difference between the radiomics and combined models. They also concluded that radiomics based on RFs obtained from CTA images can estimate TSS in patients with AIS
[29][58].
2.9. Differentiation Hemorrhage from Iodinated Contrast Extravasation after Thrombectomy
Chen X et al. in their very interesting study of 101 patients, tried to differentiate intracranial contrast extravasation from intraparenchymal hemorrhage (IPH) after mechanical thrombectomy (MT). They used RFs from non-enhanced CT (NECT) images after MT. They used manual segmentation and extracted 1316 RFs from 166 intraparenchymal areas of hyperattenuation. They obtained very interesting results, with an AUC between 0.848 and 0.826 (training and validation cohorts). The accuracy (ACC), sensitivity (S), specificity (Spec), negative predictive value (NPV) and positive predictive value (PPV) were 0.776, 0.767, 0.789, 0.682 and 0.852, respectively. They concluded that radiomics can effectively differentiate IPH from intracranial contrast extravasation after MT
[30][62].
3. Conclusions
Radiomic models can provide valuable information for the management of patients with AIS. The results obtained with radiomic models are very good and encouraging. The wide variety of clinical situations in which radiomics provides valuable information in patients with AIS suggests that scholars may be looking at a model that will change the paradigm of clinical management of patients with AIS. Further prospective studies are needed to investigate the utility of radiomics in the mentioned fields of knowledge and others that may appear in the future.