It has been shown that polyphenols in various natural sources can provide curative effects against various brain diseases and disorders through neuroprotective effects. These neuroprotective effects are mainly attributed to its ability to cross the blood-brain barrier, remove reactive oxygen species, and cause chelation of metal ions. Polyphenols increase the concentration of neurotrophic factors and bind directly to the membrane receptors of these neurotrophic factors to modulate and activate the signaling cascades that allow the plasticity, survival, proliferation, and growth of neuronal cells, allowing better learning, memory, and cognition. Furthermore, polyphenols do not have serious adverse side effects from their consumption. For more information on this topic, visiting the original article at https://www.mdpi.com/1420-3049/28/14/5415 is highly recommended.
- polyphenols
- neurodegenerative diseases
- plantas medicinales
- flavonoides
1. Introducctióon
Due to the increase in human life expectancy and the number of older adults, there is predicted to be an increase of around 50% in people aged 60 to 80 years. Thus, a third of the population will be over 65 and a quarter over 80. This is why the group of patients suffering from neurodegenerative diseases (NDs) with severe neurological deficits and dementia is growing significantly, a critical public health problem. With this enormous increase, investigations into the causes, risks, early diagnosis, and prevention of the disease, alongside its effective management, are urgently needed for the affected patients [1–4].
Polyphenols are large phytochemicals found in natural sources of plant foods, herbs, and other essential nutrients in the human diet [4–8]. Polyphenols are abundantly ingested in the human diet, where more than 1 mg of polyphenol content per serving can be consumed [8], and up to 1 g of median total polyphenol intake per day [9,10]. They contribute to food characteristics and oxidative stability of food [11,12]. Furthermore, plant polyphenols protect the plant from reactive oxygen species (ROS), ultraviolet (UV) radiation, pathogens, parasites, and plant predators [13].
The search for natural strategies to promote healthy aging drives the extensive study of plant polyphenols to prevent deterioration and age-related diseases, including NE. In vitro, cell-based, animal, and human studies have attempted to decipher the mechanisms behind the neuroprotection of dietary polyphenols.
1.1. Neurodegenerative Diseases (NDs)NDs are a group of disorders of the central nervous system (CNS), which are characterized by populations of neurons that progressively lose their functions and connections [3], resulting in sensory and motor deficits and cognitive impairment [13]. NDs are broadly classified through their clinical symptoms, the most common being extrapyramidal and pyramidal movement disorders, and cognitive or behavioral disorders [3].
In NDs, the accumulation of ubiquitin-proteasomal systems (amyloid deposits) and neurofibrillary tangles (or Lewy bodies) generates oxidative stress (ROS), excitotoxicity, neuronal and synaptic dysfunction, impairment of protein degradation systems, endoplasmic reticulum (ER) stress, mitochondrial dysfunction, DNA damage, inflammation, and re-entry into the cell cycle, eventually causing apoptosis [3,14,15,16,17,18,19,20].
The most common NDs include Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), epilepsy, Creutzfeldt–Jakob disease, and Huntington’s disease (HD). All diseases have a progressive course with clinical and biochemical changes that affect the CNS [3,20,21].
The brain captures 20% of the total oxygen consumed with a high proportion of easily peroxidizable polyunsaturated fatty acids [22,23], generating large amounts of ROS in the brain. A change in the balance between the generation of ROS and the elimination or detoxification of these species is denoted as “oxidative stress”, which is a condition associated with chronic diseases causing cell death [14]. These events are more common in the high and sustained production of ROS and reduced levels of antioxidant defenses, as occurs in various pathologies and during the process of normal aging [13,14,23].
NDs and some chronic degenerative and cardiovascular diseases increase the possibility of causing damage to the CNS, causing regional degeneration of the brain and cognitive and behavioral disorders. The link between diabetes, hypertension, cardiovascular disease, and degenerative brain disease is well established, as these diseases can cause cognitive impairment, regional brain degeneration, memory impairment, diabetic neuropathy, brain loss, and memory impairment, and may eventually cause AD, and an alteration to the blood–brain barrier (BBB), thereby promoting neuroinflammation and exacerbation of amyloid pathologies among other brain affectations [18,24,25,26,27,28,29,30].
1.2. Medicinal Plants and Secondary Metabolites
Medicinal plants are a therapeutic alternative with various pharmacological properties due to their different chemical components, which can act individually or in synergy. In addition, they are well known for having fewer or no side effects [31,32,33].
Plants produce essential compounds for survival, growth, development, and reproduction, such as sugars, proteins, amino acids, and secondary or non-essential products [33,34,35]. These secondary metabolites are essential to defend plants against biotic or abiotic stress. However, they also attract pollinators and serve as signals or regulators in plant-environment interactions [35,36,37].
Plant secondary metabolites have been widely studied for health maintenance and the prevention, diagnosis, amelioration, or treatment of physical and mental illnesses [32,38]. In the pharmaceutical industry, they are particularly interesting since they are used as drugs or in the development of new drugs [32]. In addition, they are also considered as food additives for therapeutic, aromatic, and culinary purposes, as cosmetics, chemicals, and, more recently, as nutraceuticals, which has exponentially increased their commercial importance and value [11,31,39].
Secondary metabolites are structurally and chemically diverse, meaning they are classified according to their structural and biosynthetic pathway similarities: fatty acids and polyketides (from the acetate pathway), phenylpropanoids, polyphenols, and aromatic amino acids (from the shikimate pathway), terpenoids and steroids (from the mevalonate pathway), and nitrogen-containing compounds (alkaloids) [36,37,38,40,41,42].
- Polyphenols in the Nervous System and Their Neuroprotective Effects
Polyphenols are believed to be present in low concentrations in the brain (1 nmol/g of tissue), making them sufficient to affect neuronal pathways since they can cross the BBB by diffusion, either as aglycones or as their conjugation products. The ability of flavonoids to permeate the BBB depends not only on their lipophilicity but also on their conjugation capacity; metabolites that are conjugated by methylation in the small intestine and the liver, being more lipophilic (less polar), can permeate the BBB faster than their aglycones of origin. Less lipophilic polyphenols cross the BBB when they bind to specific ATP-dependent transporters. Polyphenols can directly interact with neurotransmitters in the signaling cascades of several kinases, such as MAPK, PI3K, and PKB [13,14,21,40,54,67,68,69,70].
In recent decades, polyphenols have been exhaustively studied for their prevention and possible treatment of age-related NDs. Consequently, a wide range of polyphenols are known to influence neuronal function [4,6,47,71] and provide pleiotropic effects in neuronal cells [21]. Diets rich in polyphenols have been shown to provide benefits for maintaining cognitive functions due to the survival, differentiation, and improvement of neuronal function and regeneration [6,53,72,73,74,75]. Furthermore, polyphenols stop the progression of NDs by positively affecting memory, learning, and cognition [75]. The neuroprotective effect of flavonoids in AD is related to the mediation of glycogen synthase kinase 3β (GSK3β) and cyclin-dependent kinase 5 (CDK5) [76].
The latter is from a direct neuroprotective approach. However, polyphenols also provide indirect neuroprotective effects by modulating the composition of the intestinal microbiota and the metabolites produced, among other mechanisms. Both approaches modify the production of neurotransmitters and neuropeptides, to influence brain functions [13,55].
In the treatment of ND, there are MAO-A inhibitors for the treatment of mental disorders, such as depression and anxiety, and MAO-B inhibitors for the treatment of neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases. Flavonoids can inhibit monoamine oxidase-A and monoamine oxidase-B [77].
Polyphenols improve the regulation of neuronal survival, acting through different points of signaling pathways; this may be a promising approach for treating CNS diseases [28]. Brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), members of the neurotrophin family, are associated with the development and regeneration of neurons (neurogenesis) and long-term potentiation in the hippocampus; they also induce structural changes in synapses and survival and resistance to neuronal damage, all of which are widely attributed as a critical objective in neuroprotection through the presence of polyphenols [75,78,79].
The CNS and peripheral tissues mainly produce neurotrophins and are closely related among humans, rats, and mice [78,80]. There is a positive correlation between cognitive performance and the concentration of BDNF in the brain. In contrast, decreased BDNF production has been identified as a possible pathogenic factor in brain diseases and disorders in animals and humans [75,79,81].
The transmembrane protein tropomyosin kinase B (TrkB) receptor is a specific receptor for BDNF, while TrkA is the receptor for NGF, which is widely expressed in the mammalian brain. BDNF/NGF and TrkB/TrkA, and glial cell line-derived neurotrophic factor (GDNF) are essential for adult synaptic plasticity, memory formation, neurite outgrowth, neurotrophic activities, and the activation of neuroprotective pathways. Activation of TrkB is faster (approximately 2 min), and its deactivation occurs within 30 min. Both BDNF and polyphenols, when stimulating TrkB, activate three important downstream intracellular signaling cascades, including the PI3K/Akt, phospholipase C-γ (PLC-γ), and MAPK/ERK pathways (path-way 1 in Figure 1).
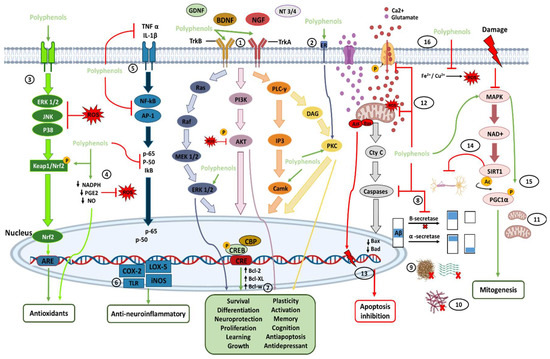
Figure 1. Direct (path-ways 1–11) and indirect (path-ways 12–16) signaling pathways of polyphenols involved in brain neuroprotection. Polyphenols provide antioxidant effects mainly through the direct and indirect elimination of ROS and through the Nrf2 pathway. Polyphenols also have anti-neuroinflammatory effects by inhibiting and modulating cytosines and proinflammatory transcriptional factors. In addition, by binding directly with neurotrophic factors and their receptors, polyphenols can activate pathways of neuronal survival, growth, proliferation, and neuroprotection, among others. Polyphenols can also control the inhibition of apoptosis and increase mitogenesis by inhibiting proapoptotic molecules and activating the MAPK pathway. In addition, polyphenols provide several indirect pathways of neuroprotection, each of which is described in more detail in the text. The numbers enclosed within the circles indicate the number of the path-way to which they refer in the text.
These signaling cascades, ultimately, lead to the phosphorylation of the cAMP response element-binding protein (CREB) and regulate transcription in neurons [53,67,78,81,82]. CREB is a cellular transcription factor in higher eukaryotes and is relatively abundant in the brain, particularly in neurons. The number of surviving neurons is closely related to the concentration of phosphorylated CREB (pCREB), which plays an essential role in learning and memory in the brain [27]. In the hippocampus, it has been shown that activation of phosphatidylinositol-3-kinase (PI3K) and its downstream effector on Akt could upregulate CREB phosphorylation, and prevent neuronal death [80,81,82,83]. BDNF/TrkB and NGF/TrkA signaling are involved in neuronal survival, memory formation, antidepressant-like effects, neural plasticity, and stress resistance. The ERK (extracellular signal-regulated kinase) pathway, a part of the mitogen-activated protein kinases (MAPKs), has been involved in various physiological functions of neurons, including proliferation, differentiation, and survival (by the induction of survival genes and the inhibition of proapoptotic proteins). ERK1/2 is activated after the phosphorylation of threonine and tyrosine residues, which changes its localization and phosphorylation of different target molecules. It has been proposed that CREB, a downstream regulator of the ERK cascade, is involved in neuronal proliferation (neurogenesis), neuroplasticity, emotion, and cognition.
Ca2+/calmodulin-dependent protein kinases (CaMKs) are a family of serine/threonine protein kinases (CaMKI, CaMKII, and CaMKIV). CaMKII is abundantly expressed at postsynaptic sites, and its activation contributes to synaptic protein phosphorylation. CaMKIV is found primarily in neuronal nuclei and is crucial for long-term memory, in the brain, by activating CREB, which stimulates the transcription of target genes by binding to the DNA cAMP response element (CRE) region. In addition, the CREB pathway positively affects cognitive health, neuronal survival, neurogenesis, synaptic plasticity, and general neuronal activation. In this regard, polyphenols can bind to the estrogen receptor (ER) and activate neurotrophic effects through protein kinase C (PKC) pathways (path-way 2 in Figure 1) [13,23,27,70,78,82,83,84,85].
On the other hand, in neuroinflammatory diseases, such as MS, the administration of polyphenols increases the production of BDNF, which may show neuroprotective activity due to its immunomodulatory action. Therefore, polyphenols can be used as a therapeutic strategy in detecting and preventing inflammatory neurological disorders and, generally, enhance neuroprotection [77,78]. Likewise, there are reports that intestinal microbes can directly affect the release of BDNF, dopamine, serotonin, GABA, catecholamines, and histamine in the brain. Thus, having a healthy microbiota can increase these molecules and lead to neuroprotective effects. [23].
For more information on this topic, visiting the original article at https://www.mdpi.com/1420-3049/28/14/5415 is highly recommended.
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85]References
- Heemels, M.T. Neurodegenerative diseases.. Nature 2016, 539, 179.
- Banjari, I.; Marˇcek, T.; Tomi´c, S.; Waisundara, V.Y. Forestalling the Epidemics of Parkinson’s Disease Through Plant-Based Remedies.. Front. Nutr. 2018, 30, 95.
- Reith, W. Neurodegenerative diseases.. Radiologe 2018, 58, 241-258.
- Rojas-García, A.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.L.; Arráez-Román, D.; Segura-Carretero, A. Neuroprotective Effects of Agri-Food By-Products Rich in Phenolic Compounds. . Nutrients 2023, 15, 449.
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders.. Mol. Neurobiol. 2011, 44, 192-201.
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.; Butler, L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. . Genes Nutr. 2009 2009, 4, 227-242.
- Gomez-Pinilla, F.; Nguyen, T.T. Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. . Nutr. Neurosci. 2012, 15, 127-133.
- Silva, R.F.M.; Pogaˇcnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. . Antioxidants 2020, 9, 61.
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs.. Pharmacogn. Rev. 2011, 5, 1-12.
- Leclerc, M.; Dudonné, S.; Calon, F. Can Natural Products Exert Neuroprotection without Crossing the Blood-Brain Barrier? . Int. J. Mol. Sci. 2021. 2021, 22, 3356.
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors.. Molecules 2018, 23, 762.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. . Oxid. Med. Cell. Longev. 2009, 2, 270.
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. . Int. J. Mol. Sci. 2020, 21, 2564.
- Campos-Esparza, M.R.; Torres-Ramos, M. Neuroprotection by Natural Polyphenols: Molecular Mechanisms. . Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 269.
- Seward, M.E.; Swanson, E.; Norambuena, A.; Reimann, A.; Cochran, J.N.; Li, R.; Roberson, E.D.; Bloom, G.S. Amyloid- signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer’s disease. . J. Cell Sci. 2013, 126, 1278.
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal Cell Death Mechanisms in Major Neurodegenerative Diseases. . Int. J. Mol. Sci. 2018, 19, 3082.
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ahmad, J.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. . Eur. J. Pharmacol. 2020, 886, 173412.
- Dugger, B.N.; Hentz, J.G.; Adler, C.H.; Sabbagh, M.N.; Shill, H.A.; Jacobson, S.; Caviness, J.N.; Belden, C.; Driver-Dunckley, E.; Davis, K.J.; et al.et al. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. . J. Neuropathol. Exp. Neurol. 2014, 73, 244.
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. . Cold Spring Harb. Perspect. Biol. 2017, 9, a028035 2017, 9, a028035.
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. I. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands. 2017, 145, 301-307.
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. . Curr. Neuropharmacol. 2017, 15, 562.
- Assunção, M.; Santos-Marques, M.J.; Carvalho, F.; Lukoyanov, N.V.; Andrade, J.P. Chronic green tea consumption prevents age-related changes in rat hippocampal formation. . Neurobiol. Aging. 2011, 32, 707.
- Zhang, Z.; Zhang, Y.; Li, J.; Fu, C.; Zhang, X. The Neuroprotective Effect of Tea Polyphenols on the Regulation of Intestinal Flora. . Molecules 2021, 26, 3692.
- Tan, X.; Fang, P.; An, J.; Lin, H.; Liang, Y.; Shen,W.; Leng, X.; Zhang, C.; Zheng, Y.; Qiu, S.; et al. Micro-structural white matter abnormalities in type 2 diabetic patients: A DTI study using TBSS analysis. . Neuroradiology 2016, 58, 1209.
- Ferris, J.K.; Inglis, J.T.; Madden, K.M.; Boyd, L.A. Brain and Body: A Review of Central Nervous System Contributions to Movement Impairments in Diabetes. . Diabetes. 2020, 69, 3-11.
- Erus, G.; Battapady, H.; Zhang, T.; Lovato, J.; Miller, M.E.; Williamson, J.D.; Launer, L.J.; Bryan, R.N.; Davatzikos, C. Spatial patterns of structural brain changes in type 2 diabetic patients and their longitudinal progression with intensive control of blood glucose. . Diabetes Care. 2015, 38, 97.
- Zhang, S.; Xue, R.; Hu, R. The neuroprotective effect and action mechanism of polyphenols in diabetes mellitus-related cognitive dysfunction. . Eur. J. Nutr. 2020, 59, 1295.
- Biessels, G.J.; Strachan, M.W.; Visseren, F.L.; Kappelle, L.J.; Whitmer, R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. . Lancet Diabetes Endocrinol. 2014, 2, 246.
- Bergantin, L.B. Hypertension, Diabetes and Neurodegenerative Diseases: Is there a Clinical Link through the Ca2+/cAMP Signalling Interaction? . Curr. Hypertens. Rev. 2019, 15, 32.
- Ungvari, Z.; Toth, P.; Tarantini, S.; Prodan, C.I.; Sorond, F.; Merkely, B.; Csiszar, A. Hypertension-induced cognitive impairment: From pathophysiology to public health. . Nat. Rev. Nephrol. 2021, 17, 639.
- Chukwuma, C.I.; Matsabisa, M.G.; Ibrahim, M.A.; Erukainure, O.L.; Chabalala, M.H.; Islam, M.S. Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: A review. . J. Ethnopharmacol. 2019, 235, 329.
- Hachkova, H.; Nagalievska, M.; Soliljak, Z.; Kanyuka, O.; Kucharska, A.Z.; Sokół-Ł˛etowska, A.; Belonovskaya, E.; Buko, V.; Sybirna, N. Medicinal Plants Galega officinalis L. and Yacon Leaves as Potential Sources of Antidiabetic Drugs. . Antioxidants. 2021, 10, 1362.
- Williams, D.H.; Stone, M.J.; Hauck, P.R.; Rahman, S.K. Why are secondary metabolites (natural products) biosynthesized? . J. Nat. Prod. 1989, 52, 1189.
- Madariaga-Mazón, A.; Hernández-Alvarado, R.B.; Noriega-Colima, K.O.; Osnaya-Hernández, A.; Martinez-Mayorga, K. Toxicity of secondary metabolites. . Phys. Sci. Rev. 2019, 4, 20180116.
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. . Plant Physiol. 2020, 184, 39.
- Arowolo, M.A.; He, J. Use of probiotics and botanical extracts to improve ruminant production in the tropics: A review. . Anim. Nutr. 2018, 4, 241.
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al.et al. Role of Promising Secondary Metabolites to Confer Resistance Against Environmental Stresses in Crop Plants: Current Scenario and Future Perspectives.. Front. Plant Sci. 2022, 13, 881032. 2022, 13, 881032.
- Debbab, A.; Aly, A.H.; Proksch, P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. .. Fungal Divers. 2011, 49, 1-12.
- Croteau, R.; Kutchan, T.; Lewis, N.G. Natural products (secondary metabolites). . In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000, 1, 1250-1319.
- Orozco, M.F.; Vázquez-Hernández, A.; Fenton-Navarro, B. Active compounds of medicinal plants, mechanism for antioxidant and beneficial effects. . Phyton 2019, 88, 1-10.
- Saldivar, J.C.; Hamperl, S.; Bocek, M.J.; Chung, M.; Bass, T.E.; Cisneros-Soberanis, F.; Samejima, K.; Xie, L.; Paulson, J.R.; Earnshaw, W.C.; et al.et al. An intrinsic S/G2 checkpoint enforced by ATR. . Science 2018, 361, 806.
- Renaud, J.; Martinoli, M.G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. . Int. J. Mol. Sci. 2019, 20, 1883.
- Zhang, W.; Zhao, W.; Wang, J.; Xu, Q.; Li, S.; Yin, C. Imaging Diagnosis of Central Nervous System Damage in Patients with T2DM. . Neurosci. Lett. 2020, 33, 135092.
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. . Mol. Plant 2010, 3, 956.
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. . Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. . Molecules 2020, 25, 3809.
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. . Annu. Rev. Plant Biol. 2012, 63, 73.
- Lamport, D.J.; Williams, C.M. Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses. . Brain Plast. 2021, 6, 139.
- Silveira, A.C.; Dias, J.P.; Santos, V.M.; Oliveira, P.F.; Alves, M.G.; Rato, L.; Silva, B.M. The Action of Polyphenols in Diabetes Mellitus and Alzheimer’s Disease: A Common Agent for Overlapping Pathologies. . Curr. Neuropharmacol. 2019, 17, 590–613. 2019, 17, 590.
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? . Antioxidants 2020, 9, 982.
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G.; et al. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. . Nutrients 2022 2022, 14, 819.
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids-food sources and health benefits. . Rocz. Panstw. Zakl. Hig. 2014, 65, 79.
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. . Antioxidants 2015, 4, 1-21.
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. . Crit. Rev. Food Sci. Nutr. 2019, 59, 2040.
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. . Int. J. Mol. Sci. 2021, 22, 3715.
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. . Nutrients 2022, 14, 5373.
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. . Mol. Nutr. Food Res. 2010, 54, 7-16.
- Czerwiecki, L. Współczesne poglady na role przeciwutleniaczy ro´slinnych w profilaktyce chorób cywilizacyjnych [Contemporary view of plant antioxidants role in prevention of civilization diseases]. . Rocz. Panstw. Zakl. Hig. 2009, 60, 201-206.
- Uno, T.; Yasui-Furukori, N. Effect of grapefruit juice in relation to human pharmacokinetic study. . Curr. Clin. Pharmacol. 2006, 1, 157-161.
- Koga, N.; Ohta, C.; Kato, Y.; Haraguchi, K.; Endo, T.; Ogawa, K.; Ohta, H.; Yano, M. In vitro metabolism of nobiletin, a polymethoxy-flavonoid, by human liver microsomes and cytochrome P450. . Xenobiotica 2011, 41, 927.
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. . Biochim. Biophys. Acta 2016, 1863, 2977.
- Kumar, R.; Kumar, R.; Khurana, N.; Singh, S.K.; Khurana, S.; Verma, S.; Sharma, N.; Vyas, M.; Dua, K.; Khursheed, R.; et al.et al. Improved neuroprotective activity of Fisetin through SNEDDS in ameliorating the behavioral alterations produced in rotenone-induced Parkinson’s model. . Environ. Sci. Pollut. Res. Int. 2022, 29, 50488.
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Ageing, neuroinflammation and neurodegeneration. Front. Biosci. 2015, 7, 189–204. Front. Biosci. 2015, 7, 189.
- Santos, V.H.; Minatel, I.O.; Lima, G.P.; Silva, R.M.; Chen, C.Y.O. Antioxidant capacity and phytochemical characterization of Spathodea campanulata growing in different climatic zones in Brazil. Biocatal. . Biocatal. Agric. Biotechnol. 2020, 24, 101536.
- Mandel, S.; Youdim, M.H. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. F. Free Radic. Biol. Med. 2004, 37, 304.
- Quiñones, M.; Miguel, M.; Aleixandre, A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular [The polyphenols, naturally occurring compounds with beneficial effects on cardiovascular disease]. . Nutr. Hosp. 2012, 27, 76.
- Bhullar, K.S.; Rupasinghe, H.P. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. . Oxid. Med. Cell. Longev. 2013, 2013, 891748.
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. . Free Radic. Biol. Med. 2004, 36, 592.
- Frade, J.G.; Ferreira, N.R.; Barbosa, R.M.; Laranjinha, J. Mechanisms of neuroprotection by polyphenols. . Cent. Nerv. Syst. Agents Med. Chem. 2005, 5, 307–318. 2005, 5, 307.
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. . Neurochem. Int. 2015, 89, 126.
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. . Genes Nutr. 2008, 3, 115.
- Bari, A.; Shah, S.M.M.; Al-Joufi, F.A.; Shah, S.W.A.; Shoaib, M.; Shah, I.; Zahoor, M.; Ahmed, M.N.; Ghias, M.; Shah, S.M.H.; et al.et al. E Effects of Artemisia macrocephala Jacquem on Memory Deficits and Brain Oxidative Stress in Streptozotocin-Induced Diabetic Mice. . Molecules 2022, 27, 2399.
- Bastianetto, S.; Zheng, W.H.; Quirion, R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: Involvement of its flavonoid constituents and protein kinase C. . J. Neurochem. 2000, 74, 2268.
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period.. Am. J. Epidemiol. 2007, 165, 1364–1371. 2007, 165, 136.
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period.. Am. J. Epidemiol. 2007, 165, 1364.
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. A. Arch. Med. Sci. 2015, 11, 1165.
- Zeng, P.; Fang, M.; Zhao, H.; Guo, J. A network pharmacology approach to uncover the key ingredients in Ginkgo Folium and their anti-Alzheimer’s disease mechanisms. . Aging 2021, 13, 18993.
- Baek, S.C.; Park, M.H.; Ryu, H.W.; Lee, J.P.; Kang, M.G.; Park, D.; Park, C.M.; Oh, S.R.; Kim, H. Rhamnocitrin isolated from Prunus padus var. seoulensis: A potent and selective reversible inhibitor of human monoamine oxidase A. . Bioorg. Chem. 2019, 83, 317.
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. . Growth Factors 2004, 22, 123.
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. . Int. J. Mol. Sci. 2020, 21, 841.
- Zhen, Y.F.; Zhang, J.; Liu, X.Y.; Fang, H.; Tian, L.B.; Zhou, D.H.; Kosten, T.R.; Zhang, X.Y. Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. . Psychopharmacology 2013, 227, 93.
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. . Drug Des. Devel. Ther. 2015, 10, 23-42.
- Mohammadi, A.; Amooeian, V.G.; Rashidi, E. Dysfunction in Brain-Derived Neurotrophic Factor Signaling Pathway and Susceptibility to Schizophrenia, Parkinson’s and Alzheimer’s Diseases. . Curr. Gene Ther. 2018, 18, 45-63.
- Srivastava, P.; Dhuriya, Y.K.; Kumar, V.; Srivastava, A.; Gupta, R.; Shukla, R.K.; Yadav, R.S.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K.; et al. PI3K/Akt/GSK3 induced CREB activation ameliorates arsenic mediated alterations in NMDA receptors and associated signaling in rat hippocampus: Neuroprotective role of curcumin. . Neurotoxicology 2018, 67, 190.
- Ye, S.; Xie, D.J.; Zhou, P.; Gao, H.W.; Zhang, M.T.; Chen, D.B.; Qin, Y.P.; Lei, X.; Li, X.Q.; Liu, J.; et al.et al. Huang-Pu-Tong-Qiao Formula Ameliorates the Hippocampus Apoptosis in Diabetic Cognitive Dysfunction Mice by Activating CREB/BDNF/TrkB Signaling Pathway. . Evid. Based Complement. Altern. Med. 2021, 2021, 5514175.
- Bhakkiyalakshmi, E.; Dineshkumar, K.; Karthik, S.; Sireesh, D.; Hopper,W.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene-mediated Nrf2 activation: Mechanistic insights on Keap1:Nrf2 interface. . Bioorg. Med. Chem. 2016, 24, 3378-3386.
