Polyphenols, a class of bioactive compounds, including flavonoids, phenolic acids, and lignans, are commonly found in plant-based diets with a variety of biological actions, including antioxidant, anti-inflammatory, and anticancer effects. Unfortunately, polyphenols are not widely used in nutraceuticals since many of the chemicals in polyphenols possess poor oral bioavailability. Thankfully, polyphenols can be encapsulated and transported using bio-based nanocarriers, thereby increasing their bioavailability. Polyphenols’ limited water solubility and low bioavailability are limiting factors for their practical usage, but this issue can be resolved if suitable delivery vehicles are developed for encapsulating and delivering polyphenolic compounds.
- polyphenols
- bioavailability
- nanocarriers
1. Introduction
2. Polyphenol Bioavailability
2. Polyphenol Bioavailability
4. Nanoformulations Made from Dietary Macromolecules to Encapsulate and Transport Polyphenols
3. Nanoformulations Made from Dietary Macromolecules to Encapsulate and Transport Polyphenols
4.1. Nanoformulations of Casein
3.1. Nanoformulations of Casein
4.2. Nanoformulations of Gelatin
3.2. Nanoformulations of Gelatin
4.3. Nanoformulations of Polysaccharides in Food
3.3. Nanoformulations of Polysaccharides in Food
4.4. Nanoformulations with a Protein–Polysaccharide Conjugate
3.4. Nanoformulations with a Protein–Polysaccharide Conjugate
The Maillard process produces polysaccharide glycosylated proteins that limit protein precipitation induced by the high concentration or contact with polyphenols, which are polyphenol encapsulation materials [91][41]. The protein core of Maillard-synthesized gelatin–dextran conjugate nanoparticles included tea polyphenols. EGCG-loaded conjugate nanoparticles had an average diameter of 86 nm and limited distribution under optimum circumstances. EGCG has a 360 wt.% loading capacity and pH-independent encapsulation efficiency. Encapsulated EGCG had equivalent or higher cytotoxicity against MCF-7 cells than free EGCG in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [92][42].4.5. Nanoformulations Derived from Dietary Lipids
3.5. Nanoformulations Derived from Dietary Lipids
Solid lipid nanoparticles (SLNs) improve lipid-soluble polyphenol solubility and bioavailability. In a coculture system of absorptive Caco-2 and mucus-secreting HT29-MTX cells, SLN’s curcumin delivery was tested, and it was found that curcumin encapsulated in SLN delivered better than unencapsulated curcumin without affecting cellular junction integrity [94][43]. Another study [95][44] found that curcumin-loaded SLNs could prolong in vitro anticancer efficacy, cellular absorption, and in vivo bioavailability. Resveratrol was loaded and encapsulated in two forms of SLN [96][45]. The nanoparticle crystal structure was changed by resveratrol, suggesting its entrapment. During incubation in digestive fluids, resveratrol mainly stayed with lipid nanoparticles [96][45]. A study [97][46] created glyceryl behenate-based solid SLNs to encapsulate and distribute resveratrol. Resveratrol-loaded SLNs were as effective as free resveratrol as an anticancer drug in a cytotoxicity experiment. In a Wistar rat bio-distribution investigation, SLNs increased brain resveratrol content by p < 0.001 [97][46]. Resveratrol-loaded stearic-acid-based SLNs coated with poloxamer 188 were successfully synthesized utilizing solvent diffusion–solvent evaporation and showed extended drug release in vitro up to 120 h. Compared to the solution, the lipid formulation improved the oral bioavailability of resveratrol by eight-fold [98][47]. After loading into SLNs, resveratrol solubility, stability, and intracellular delivery increased. With or without resveratrol, SLNs below 180 nm loading went quickly across the cell membrane, diffused throughout the cytoplasm, migrated sequentially among cellular levels, and localized in the perinuclear region without cytotoxicity. Resveratrol in solution was less cytostatic than SLN–resveratrol. Resveratrol’s cell-proliferation-reducing effects may be enhanced by SLN delivery [99][48]. Therefore, it can be concluded that nanoformulations based on dietary lipids can be treated as a reliable vehicle delivery for polyphenols.54. Nanoformulations of Polyphenols and Therapeutic Properties
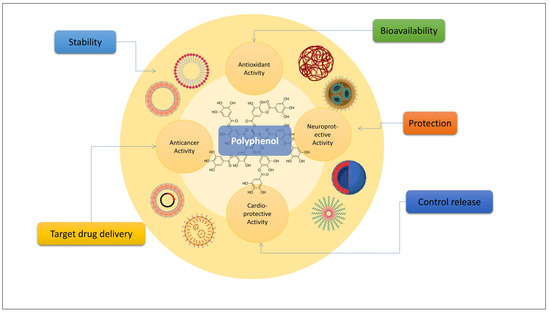
5.1. Cardioprotective Effects
4.1. Cardioprotective Effects
Oxidative stress is thought to be a major factor in the development of cardiovascular disease. Increased oxidative stress and a diminished antioxidant reserve are associated with both acute and chronic heart failure [100][49]. Cardiovascular disease, especially ischemic heart disease, and stroke due to atherosclerosis are among the oxidative-related disorders that polyphenols can protect. Polyphenols have been shown to protect the heart against oxidative stress-related diseases in a number of studies [101,102][50][51]. A bioactive polymer (PLGA layer) was deposited on top of a superparamagnetic SiN.SiN@QC-PLGA nano-bio-composite [103][52] to modify the drug discharge profile and increase the functional resemblance to the local myocardial by facilitating the cell recruitment, expansion, attachment, and articulation of cardiac proteins. The effectiveness of recently produced nano-formulated natural therapies against hypertension, atherosclerosis, thrombosis, and myocardial infarction has been studied [104][53].5.2. Neuroprotective Effects
4.2. Neuroprotective Effects
Researchers are studying cerium oxide nanoparticles because they show promising molecules as a treatment for several neurological illnesses [109][54]. The neuroprotective effects of CeO2@SiO2-PEG nanoparticles (CSP-NPs) for proanthocyanidin and curcumin delivery have been studied [110][55]. Hydrophilic curcumin (Cur) and hydrophobic proanthocyanidin (PAC) were, respectively, loaded onto CeO2@SiO2-PEG nanoparticles to produce Cur-NPs and PAC-NPs. Cur-NPs and PAC-NPs inhibited acetylcholinesterase (AchE) activity and protected neurons against A1-42-mediated toxicity in PC-12 cells. Several studies have reported different nanoparticle systems loaded with curcumin. These systems include poly (-caprolactone) (PCL), poly (lactide-co-glycolide) (PLGA), and methoxy poly (ethylene glycol) poly (-caprolactone) (MPEG-PCL). Inhibiting enzymatic and pH degradation of curcumin and showcasing its neuroprotective capabilities [111][56] are both made possible through the incorporation of curcumin into nanoparticle systems. A safe and efficient therapeutic approach for the treatment of Alzheimer’s disease may be the development of PEGylated PLGA nanoparticles loaded with two medicines (EGCG and acetyl acid). When mice were orally administered EGCG/ascorbic acid NPs, the compound accumulated throughout the brain and other important organs. This formulation has been shown to have the potential to increase the drug’s persistence in both the blood and the brain [112][57]. Research has found that 4-hydroxyisophthalic acid (4-HIA)-encapsulated PLGA-NPs significantly reduced the cytotoxicity of H2O2 in PC12 cells [113][58]. Thus, the neuroprotective effects are well established using nanoformulations and polyphenols.
5.3. Cancer Treatment
4.3. Cancer Treatment
Cancer is defined as an uncontrolled growth of cells, which can lead to malignancies that are both potentially fatal and extremely costly for patients and the healthcare system. Many different diseases have traditionally been treated and prevented with natural polyphenols. Hence, due to their anticancer effects, these phytochemicals may be used as chemotherapeutic and chemopreventive drugs in a variety of malignancies [114][59]. The cytotoxic effects of curcumin-loaded PLGA nanoparticles coupled with anti-P-glycoprotein were studied in human cervical cancer KB-3-1 and KB-V1 cells, and the results showed increased curcumin solubility and cellular absorption, as well as decreased cell survival [115][60]. To activate PTT-assisted ferrous therapy in the treatment of cancer, the authors of [116][61] designed ferric-coordinated polyphenol nanoparticles. Ellagic acid loaded with schizophyllan and chitin nanoparticles exhibits anticancer effects [117][62] in MCF-7 breast cancer cells. Viability assays showed that MCF-7 cells were significantly inhibited in their ability to proliferate, with the impact being amplified at higher concentrations. The nanoencapsulation of quercetin and curcumin in a casein-based model was recently described [118][63], and these compounds were evaluated against MCF-7 cell lines.65. Polyphenol-Based Nanoformulations: Takeaway Message
Several important concerns need to be addressed in the future to speed up the development and clinical translation of polyphenol-containing nanoformulations: (a) The manufacture of polyphenol-containing nanoformulations requires the development of simple and generic methodologies, as well as the actualization of rational design and on-demand synthesis. It is important to learn more about the use of the materials’ qualities and functionalities. In their current form, nanoformulations containing polyphenols have a number of drawbacks, including a lack of physiological stability/biodegradability, drug encapsulation/loading efficiency, stimulus responsiveness, traceability, and active targeting ability. Some fresh perspectives could emerge from combining chemical grafting with supramolecular self-assembly. (b) Cancer combination therapy has found an excellent new platform in polyphenol-containing nanoformulations. Better anticancer tactics may be attainable through the development of multifunctional polyphenol-based nanoplatforms that integrate various cancer therapeutic modalities (such as chemotherapy, radiation, and immunotherapy). Nanoformulations containing polyphenols have many potential medical uses, but further research is needed. While most research so far has been on cancer treatment, polyphenols’ many health benefits also make them useful in preventing and managing bacterial infection, neurological diseases, cardiovascular conditions, diabetes, and others. Furthermore, it is anticipated that the polyphenol-containing nanoformulations will be able to include different enzymes for biocatalysis purposes. Imaging agents (fluorescent probes, MRI agents, radioactive agents) should be incorporated into various illness therapies with increased focus. (c) Systematic assessments of the biosafety and in vivo destiny of polyphenol-containing nanoformulations are also crucial. Research into polyphenol-containing nanoformulations should focus on their targeting abilities to tumor or inflamed tissues, long-term toxicity, in vivo biodegradability, renal clearance, and interaction processes with biological systems.76. Conclusions
The phenolic compounds (EGCG, resveratrol, curcumin, quercetin) found in plants in high concentrations perform a wide range of beneficial biological functions. Furthermore, poor stability, poor solubility, and limited bioavailability significantly limit the utilization of these compounds in food and medicine. Nanoparticle encapsulation not only allows for more precise targeting and controllable release but also allows for the circumvention of these limits. Nanotechnology provides an ideal carrier system for increasing the pharmacokinetics and bioavailability of polyphenols. Nanoparticles are nearly ideal as carriers; however, their side effects and toxicity must be considered and mitigated before they may be used in a therapeutic setting. As polyphenols are natural compounds that must be taken for an extended period of time in the treatment and prevention of diseases, it is vital to understand the dangerous side effects linked to the buildup of nanoparticles in the physiological system. Specifically, if the nanoparticles have a poor encapsulation rate, this will be the case. Thus, standardized in vitro and in vivo models must be constructed, and in vivo safety testing procedures must be verified to support the development and implementation of innovative, effective nanoparticles beneficial to human health.References
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in research on interactions between polyphenols and biology-based nano-delivery systems and their applications in improving the bioavailability of polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500.
- Farhan, M.; Rizvi, A.; Aatif, M.; Ahmad, A. Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites 2023, 13, 481.
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713.
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212.
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470.
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another look at dietary polyphenols: Challenges in cancer prevention and treatment. Curr. Med. Chem. 2022, 29, 1061–1082.
- Ticinesi, A.; Mancabelli, L.; Carnevali, L.; Nouvenne, A.; Meschi, T.; Del Rio, D.; Ventura, M.; Sgoifo, A.; Angelino, D. Interaction between diet and microbiota in the pathophysiology of alzheimer’s disease: Focus on polyphenols and dietary fibers. J. Alzheimer’s Dis. JAD 2022, 86, 961–982.
- Kosmalski, M.; Pekala-Wojciechowska, A.; Sut, A.; Pietras, T.; Luzak, B. Dietary intake of polyphenols or polyunsaturated fatty acids and its relationship with metabolic and inflammatory state in patients with type 2 diabetes mellitus. Nutrients 2022, 14, 1083.
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Battino, M.; et al. The effect of dietary polyphenols on vascular health and hypertension: Current evidence and mechanisms of action. Nutrients 2022, 14, 545.
- Macena, M.L.; Nunes, L.; da Silva, A.F.; Pureza, I.; Praxedes, D.R.S.; Santos, J.C.F.; Bueno, N.B. Effects of dietary polyphenols in the glycemic, renal, inflammatory, and oxidative stress biomarkers in diabetic nephropathy: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2022, 80, 2237–2259.
- Cheng, Z.; Wang, Y.; Li, B. Dietary polyphenols alleviate autoimmune liver disease by mediating the intestinal microenvironment: Challenges and hopes. J. Agric. Food Chem. 2022, 70, 10708–10737.
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189.
- Rudrapal, M.; Mishra, A.K.; Rani, L.; Sarwa, K.K.; Zothantluanga, J.H.; Khan, J.; Kamal, M.; Palai, S.; Bendale, A.R.; Talele, S.G.; et al. Nanodelivery of Dietary Polyphenols for Therapeutic Applications. Molecules 2022, 27, 8706.
- Guan, T.; Zhang, Z.; Li, X.; Cui, S.; McClements, D.J.; Wu, X.; Chen, L.; Long, J.; Jiao, A.; Qiu, C.; et al. Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems. Foods 2022, 11, 156.
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274.
- Dinu, M.; Tristan Asensi, M.; Pagliai, G.; Lotti, S.; Martini, D.; Colombini, B.; Sofi, F. Consumption of ultra-processed foods is inversely associated with adherence to the Mediterranean diet: A cross-sectional study. Nutrients 2022, 14, 2073.
- Negrati, M.; Razza, C.; Biasini, C.; Di Nunzio, C.; Vancini, A.; Dall’Asta, M.; Lovotti, G.; Trevisi, E.; Rossi, F.; Cavanna, L. Mediterranean diet affects blood circulating lipid-soluble micronutrients and inflammatory biomarkers in a cohort of breast cancer survivors: Results from the SETA study. Nutrients 2021, 13, 3482.
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270.
- Ting, Y.; Jiang, Y.; Ho, C.T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128.
- Bell, L.N. Stability testing of nutraceuticals and functional foods. In Handbook of Nutraceuticals and Functional Foods; Wildman, R.E.C., Ed.; CRC Press: New York, NY, USA, 2002; pp. 523–538.
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884.
- McClements, D.J.; Li, F.; Xiao, H. The nutraceutical bioavailability classification scheme: Classifying nutraceuticals according to factors limiting their oral bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327.
- Gonçalves, R.F.; Martins, J.T.; Duarte, C.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291.
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Designing excipient emulsions to increase nutraceutical bioavailability: Emulsifier type influences curcumin stability and bioaccessibility by altering gastrointestinal fate. Food Funct. 2015, 6, 2475–2486.
- Liu, X.; Bi, J.; Xiao, H.; McClements, D.J. Enhancement of nutraceutical bioavailability using excipient nanoemulsions: Role of lipid digestion products on bioaccessibility of carotenoids and phenolics from mangoes. J. Food Sci. 2016, 81, 754–761.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278.
- Jiménez-Monreal, A.M.; García-Diz, L.; Martínez-Tomé, M.; Mariscal, M.; Murcia, M.A. Influence of cooking methods on antioxidant activity of vegetables. J. Food Sci. 2009, 74, H97–H103.
- Rothwell, J.A.; Medina-Remón, A.; Pérez-Jiménez, J.; Neveu, V.; Knaze, V.; Slimani, N.; Scalbert, A. Effects of food processing on polyphenol contents: A systematic analysis using Phenol-Explorer data. Mol. Nutr. Food Res. 2015, 59, 160–170.
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959.
- Korus, A.; Lisiewska, Z. Effect of preliminary processing and method of preservation on the content of selected antioxidative compounds in kale (Brassica oleracea L. var. acephala) leaves. Food Chem. 2011, 129, 149–154.
- Vergara-Balderas, F.T. Canning: Process of Canning. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldra, F., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 628–632.
- Chaovanalikit, A.; Wrolstad, R.E. Anthocyanin and Polyphenolic Composition of Fresh and Processed Cherries. J. Food Sci. 2004, 69, FCT73–FCT83.
- Tucker, G.S. Food Biodeterioration and Preservation; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 81–135.
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release Off. J. Control. Release Soc. 2013, 172, 1075–1091.
- Rosales, T.K.O.; Fabi, J.P. Valorization of polyphenolic compounds from food industry by-products for application in polysaccharide-based nanoparticles. Front. Nutr. 2023, 10, 1144677.
- Hendawy, O.M. Nano-delivery systems for improving therapeutic efficiency of dietary polyphenols. Altern. Ther. Health Med. 2021, 27, 162–177.
- Rashidinejad, A.; Nieuwkoop, M.; Singh, H.; Jameson, G.B. Assessment of Various Food Proteins as Structural Materials for Delivery of Hydrophobic Polyphenols Using a Novel Co-Precipitation Method. Molecules 2023, 28, 3573.
- Shutava, T.G.; Balkundi, S.S.; Vangala, P.; Steffan, J.J.; Bigelow, R.L.; Cardelli, J.A.; O’Neal, D.P.; Lvov, Y.M. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 2009, 3, 1877–1885.
- Kumar, A.; Kurmi, B.D.; Singh, A.; Singh, D. Potential role of resveratrol and its nano-formulation as anti-cancer agent. Explor. Target. Anti-Tumor Ther. 2022, 3, 643–658.
- Chen, Y.; Liu, Y.; Dong, Q.; Xu, C.; Deng, S.; Kang, Y.; Fan, M.; Li, L. Application of functionalized chitosan in food: A review. Int. J. Biol. Macromol. 2023, 235, 123716.
- Srivastava, N.; Choudhury, A.R. Microbial polysaccharide-based nanoformulations for nutraceutical delivery. ACS Omega 2022, 7, 40724–40739.
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A review on polymer and lipid-based nanocarriers and its application to nano-pharmaceutical and food-based systems. Front. Nutr. 2021, 8, 783831.
- McClements, D.J.; Öztürk, B. Utilization of Nanotechnology to Improve the Handling, Storage and Biocompatibility of Bioactive Lipids in Food Applications. Foods 2021, 10, 365.
- Huang, Y.; Zhan, Y.; Luo, G.; Zeng, Y.; McClements, D.J.; Hu, K. Curcumin encapsulated zein/caseinate-alginate nanoparticles: Release and antioxidant activity under in vitro simulated gastrointestinal digestion. Curr. Res. Food Sci. 2023, 6, 100463.
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633.
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics 2023, 15, 746.
- Jain, A.; Sharma, T.; Kumar, R.; Katare, O.P.; Singh, B. Raloxifene-loaded slns with enhanced biopharmaceutical potential: Qbd-steered development, in vitro evaluation, in vivo pharmacokinetics, and ivivc. Drug Deliv. Transl. Res. 2022, 12, 1136–1160.
- Astley, C.; Houacine, C.; Zaabalawi, A.; Wilkinson, F.; Lightfoot, A.P.; Alexander, Y.; Whitehead, D.; Singh, K.K.; Azzawi, M. Nanostructured Lipid Carriers Deliver Resveratrol, Restoring Attenuated Dilation in Small Coronary Arteries, via the AMPK Pathway. Biomedicines 2021, 9, 1852.
- Ng, M.L.; Ang, X.; Yap, K.Y.; Ng, J.J.; Goh, E.C.H.; Khoo, B.B.J.; Richards, A.M.; Drum, C.L. Novel oxidative stress biomarkers with risk prognosis values in heart failure. Biomedicines 2023, 11, 917.
- Anupama, S.K.; Ansari, M.A.; Anand, S.; Sowbhagya, R.; Sultana, S.; Punekar, S.M.; Ravikiran, T.; Alomary, M.N.; Alghamdi, S.; Qasem, A.H.; et al. Decalepishamiltonii and its bioactive constituents mitigate isoproterenol-induced cardiotoxicity in aged rats. S. Afr. J. Bot. 2021, 151, 25–33.
- Oudot, C.; Gomes, A.; Nicolas, V.; Le Gall, M.; Chaffey, P.; Broussard, C.; Calamita, G.; Mastrodonato, M.; Gena, P.; Perfettini, J.L.; et al. CSRP3 mediates polyphenols-induced cardioprotection in hypertension. J. Nutr. Biochem. 2019, 66, 29–42.
- Wang, L.; Feng, M.; Li, Y.; Du, Y.; Wang, H.; Chen, Y.; Li, L. Fabrication of superparamagnetic nano-silica@ quercetin-encapsulated PLGA nanocomposite: Potential application for cardiovascular diseases. J. Photochem. Photobiol. B. 2019, 196, 111508.
- Hesari, M.; Mohammadi, P.; Khademi, F.; Shackebaei, D.; Momtaz, S.; Moasefi, N.; Farzaei, M.H.; Abdollahi, M. Current Advances in the Use of Nanophytomedicine Therapies for Human Cardiovascular Diseases. Int. J. Nanomed. 2021, 16, 3293–3315.
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2017, 9, e1444.
- Chen, Y.; Zhang, R.; Xie, B.; Sun, Z.; McClements, D.J. Lotus seedpod proanthocyanidin-whey protein complexes: Impact on physical and chemical stability of β-carotene-nanoemulsions. Food Res. Int. 2020, 127, 108738.
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460.
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75.
- Ravikiran, T.; Anand, S.; Ansari, M.A.; Alomary, M.N.; AlYahya, S.; Ramachandregowda, S.; Alghamdi, S.; SindhghattaKariyappa, A.; Dundaiah, B.; Madhugiri Gopinath, M.; et al. Fabrication and in vitro Evaluation of 4-HIA Encapsulated PLGA Nanoparticles on PC12 Cells. Int. J. Nanomed. 2021, 16, 5621–5632.
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702.
- Punfa, W.; Yodkeeree, S.; Pitchakarn, P.; Ampasavate, C.; Limtrakul, P. Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol. Sin. 2012, 33, 823–831.
- Yu, X.; Shang, T.; Zheng, G.; Yang, H.; Li, Y.; Cai, Y.; Xie, G.; Yang, B. Metal-polyphenol-coordinated nanomedicines for Fe (II) catalyzed photoacoustic-imaging guided mild hyperthermia-assisted ferrous therapy against breast cancer. Chin. Chem. Lett. 2022, 33, 1895–1900.
- Pirzadeh-Naeeni, S.; Mozdianfard, M.R.; Shojaosadati, S.A.; Khorasani, A.C.; Saleh, T. A comparative study on schizophyllan and chitin nanoparticles for ellagic acid delivery in treating breast cancer. Int. J. Biol. Macromol. 2020, 144, 380–388.
- Ghayour, N.; Hosseini, S.M.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.R.; Gahruie, H.H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocoll. 2019, 87, 394–403.
