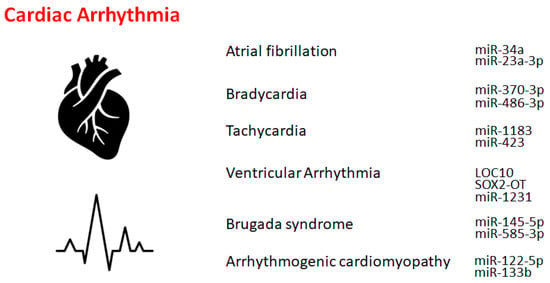
Atrial fibrillation is an asymmetrical heart rhythm that might commonly lead to heart palpitations. Interestingly, individuals carrying the non-coding 4q25 locus near the
PITX2 gene were 60% more susceptible to this abnormal heart rhythm
[8][13]. Similarly, lncRNAs may be potentially associated with the development of atrial fibrillation. Several lncRNAs, including
LINC00844,
RP11-532N4.2,
UNC5B-AS1,
RP3-332B22.1,
RP11-432J24.5, and
RP11-557H15.4, have been shown to be differentially expressed in patients with atrial fibrillation compared to normal individuals, whose target might be related to the calcium signaling pathway and/or toll-like receptor signaling pathway
[9][10][14,15]. In addition, several miRNAs, including
miR-135a, might be involved in the process of atrial fibrillation. The physiological roles of these differentially expressed ncRNAs might be associated with the pathogenesis of atrial fibrillation, which might provide a new therapeutic target and/or a prognosis marker for patients with atrial fibrillation
[9][14].
Bradycardia might be a heart dysfunction described by an extremely decreased heart rate, typically lower than 50 beats/min. Key etiological factors of bradycardia may include the dysregulation of sinus, atrial, or junctional bradycardia and/or an intricate transmission system with atrioventricular block. The most frequent form of bradycardia occurs asymptomatically during sleep and/or in athletes
[11][20]. The only treatment for insistent bradycardia may be the placement of an everlasting pacemaker. Bradycardia is usually regulated by a range of molecules, including ncRNAs, at molecular levels. Transcription factors might also control the expression of genes involved in several bradycardias by modulating a variety of effectors, including miRNAs. For example, it has been demonstrated that the function of
miR-370-3p could contribute to the development of bradycardia
[12][21], which might be triggered via the reduced function of sinus node pacemaker channels with a related reduction of the ionic current. The
miR-370-3p could directly bind to the mRNA of HCN4 to suppress its activity
[12][21]. Similarly,
miR-486-3p could suppress HCN4, inducing sinus node dysregulation such as bradycardia
[13][14][22,23]. Supraventricular arrhythmia may be an example of tachycardia that begins in the upper compartments of the heart. Tachycardia is known to be considered a fast heartbeat, which might also be regulated by a range of molecules, including ncRNAs, at molecular levels.
Ventricular arrhythmias have been described as irregular heartbeats that may derive from the ventricles. The origin of ventricular arrhythmias might be sympathetic remodeling that originates in myocardial infarction. Various ncRNAs are important regulators of inflammation and/or sympathetic remodeling following myocardial infarction. Several lncRNAs are potentially implicated in ventricular arrhythmias following myocardial infarction. For example, the lncRNA
LOC100911717 (LOC10) might be increased in cardiac cells and macrophages in the infarcted heart area
[15][26]. Another important factor in ventricular arrhythmias may be NLRP3 inflammasomes. It has been demonstrated that the role of
SOX2-OT lncRNA in regulating NLRP3 inflammasome-mediated ventricular arrhythmias. The levels of
SOX2-OT and NLRP3 inflammasomes could be intensified after ventricular arrhythmias
[16][27]. Similarly,
miR-2355-3p and
miR-1231 may play key roles in the regulation of ventricular arrhythmias
[17][18][28,29], which might provide a potential diagnostic marker as well as a potential target for various treatments
[17][28].
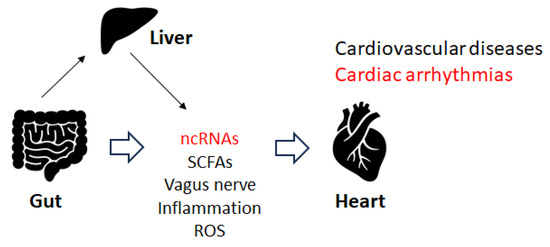
Disruption of the gut microbiota could induce cardiorespiratory morbidity. Consistently, modulation of autonomic homeostasis via the gut microbiota-brain axis could control the heart rate, independent of carotid body plasticity
[20][41]. Sympathetic neuronal communication between the hypothalamic paraventricular nucleus and the gut might also be involved in the regulation of blood pressure
[20][41]. The adult gut system might be an extremely diverse and dynamic ecosystem
[21][22][42,43], which might employ specific physiological functions including immune regulation, gut mucosal protection, and conservation of nutritional metabolisms, in addition to the development of several diseases
[23][44]. Some elements of the gut microbiota could stimulate the afferent fiber of the vagus nerve through the gut endocrine cells, which could also inspire the central nervous system (CNS). Depending on the material, the different SCFAs produced by the gut microbiota could activate the vagal afferent fibers in several ways. For example, butyric acid, a short fatty acid, directly affects afferent terminals
[24][25][45,46]. An increase in the concentration of butyric acid in the colon may produce a significant hypotensive effect, which depends on afferent colonic vagus nerve signaling and GPR41/43 receptors
[25][46]. Several investigations have considered the effect of food components on gut microbiota, which could be an imperative target for the forthcoming treatment of cardiac arrhythmias through the alteration of gut microbiota. For example, patients with arrhythmias may prefer to obtain more energy from animal fat
[26][47]. Therefore, patients with cardiac arrhythmias may be frequently diagnosed with atherosclerosis. In addition, diabetes may also be a frequent co-morbidity in individuals with arrhythmias. Furthermore, the interaction between some drugs and their impact on the gut microbiota may result in uncontrolled arrhythmias
[27][28][29][48,49,50]. Recent increasing data suggest that regulatory non-coding RNAs such as miRNAs, circular RNAs, and lncRNAs may affect host-microbe interactions. These ncRNAs have also been suggested as potential biomarkers in microbiome-associated disorders with a direct cross-talk between microbiome composition and ncRNAs
[30][51]. Therefore, gut microbiota could affect responses to stimuli by host cells with modifications to their epigenome and/or gene expression. Recent data suggest that regulatory ncRNAs such as miRNAs, circular RNAs, and lncRNAs might affect host-microbe interactions. For example, miR-155 has been associated with inflammatory bowel diseases and might also be involved in cardiac remodeling following acute myocardial infarction
[30][51]. Epigenetically,
miR-23a-3p could lead to a Th17/Treg imbalance and participate in the progression of Graves’ disease
[31][52], which might also be involved in the mechanism underlying atrial fibrillation. Consequently, gut microbiota could play a vital role in the pathogenesis of both atrial fibrillation and Graves’ disease
[31][52]. The imbalance of Th17/Treg cells induced by the alteration of gut microbiota could also play a dynamic role in the pathogenesis of arrhythmias
[32][33][53,54], suggesting that the immune response and arrhythmias are closely related. In particular, Th17 cells might be involved in the pathogenesis of atrial fibrillation, since increased levels of Th17-associated cytokines may be independently associated with an increased risk of atrial fibrillation
[32][53]. The balance between Th17 and Treg cells has been deliberated to represent a paradigm for several inflammatory and auto-immune diseases. The inflammatory response might play a key role in the pathogenesis of atrial fibrillation, and the restoration of the Th17/Treg balance might represent a promising therapeutic target for treatment
[33][54]. Interestingly, altered composition of the gut microbiota could change the expression of hepatic
miR-34a [34][55], which might also be involved in the development of atrial fibrillation. In addition, there is a negative correlation between
miR-122-5p and intestinal bacteria, including
Bacteriodes. uniformis and
Phascolarctobacterium. Faecium has been shown
[35][56], suggesting that the crosstalk between miRNA and certain gut microbiota could regulate intracellular signal transduction by controlling the expression of key genes related to the development of arrhythmias
[35][56]. As mentioned before,
miR-122-5p could possess great diagnostic potential in arrhythmogenic cardiomyopathy patients
[36][37][40,57] (
Figure 2).