Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Satoru Matsuda and Version 2 by Peter Tang.
The APRO family members may be involved in the regulation of cell growth, migration, and/or invasion. Although an APRO protein could suppress the invasiveness of several cancer cells, it has been reported that overexpression of the same APRO protein could also promote the invasiveness and/or metastasis of the same cancer cells. In general, the invasiveness of cancer cells might be associated with the function of matrix metalloproteinases (MMPs) as well as with the function of certain exosomes. However, it has been shown that exosomes involving particular APRO proteins, MMPs, and/or microRNA could contribute to the regulation of invasiveness.
- APRO protein
- microRNA
- matrix metalloproteinase
- tissue inhibitors of metalloproteinase
- exosome
1. Introduction
The APRO family is composed of at least six distinct members in vertebrates, namely, Tob1, Tob2, BTG1, BTG2/TIS21/PC3, ANA/Tob5/BTG3, and PC3b [1]. The main characteristic of this family is the presence of a highly conserved, 110-amino-acid N-terminal region, designated as the APRO homology domain [1], which holds two highly homologous regions, designated Box-A and Box-B [1][2][1,2]. Box-A has been suggested to play an antiproliferative role [2]. All the APRO family members may be involved in the regulation of cell proliferation, and actually, have the potential to regulate tumor cell growth [1][3][1,3]. Tob1 was isolated as a protein associating with the ErbB2 receptor protein [3]. Subsequently, Tob2 was isolated on the basis of its similarity to Tob1 [4]. Other family members had been identified using several different strategies [5]. A significant association has been identified between the expression level of Tob1 and clinicopathological characteristics, including the depth of invasion and/or the lymph node metastasis stage [6]. The downregulated expression of Tob1 has been found in malignant gastric cancer, suggesting that the low expression of Tob1 may be an independent indicator of poor prognosis in gastric cancers [7]. Similarly, the downregulation of Tob1 may be associated with the shorter survival of gastric cancer patients [8]. Consistently, Tob1 overexpression could not only increase the expression of PTEN, but also regulate the downstream effectors in the PI3K/PTEN signaling pathway, leading to the suppression of cancer cell proliferation [9]. In addition, significant prognostic effects of the whole APRO family have been found in lung adenocarcinoma and ovarian, colorectal, and brain cancers, but not in squamous-cell lung carcinoma [10]. Thus, accumulating evidence has shown that APRO family proteins may function as a tumor suppressor.
2. Exosomes with APRO Proteins and/or Certain MicroRNAs May Contribute to Cancer Invasion
Exosomes are a class of extracellular membrane vesicles with a circular lipid bilayer ranging in diameter from about 30 to 150 nm [11][19], which are capable of mediating invasion and/or metastasis by transferring their contents, including proteins, lipids, and nucleic acids, to adjacent cells [12][20]. Exosomes are broadly distributed in blood cells, dendritic cells, tumor cells, and other cells [13][21], which can be used for diagnosis and/or prognosis within cancer patients [14][22]. Molecular machineries of prevailing biogenesis, cargo loading, and/or delivery of exosomes may be intricate and are still under investigation. Exosomes secreted from gastric cancer cells overexpressing Tob1 could induce autophagy of LC3-II accumulation in the gastric cancer cells [15][23], which may influence the proliferation, migration, and invasion of cancer cells [16][24]. It has been shown that exosomes containing the BTG1 protein are present in the pleural fluid obtained from patients suffering from mesothelioma, lung cancer, breast cancer, and ovarian cancer [17][25]. The BTG1 protein has been also identified in plasma exosomes. In addition, the plasma-exosome-derived BTG-1 levels have been related to tumor diameter, stage, tumor metastasis, the degree of tumor differentiation, and abnormal CEA levels, in accordance with previous findings of BTG-1 expression in other cancers [17][25]. Furthermore, a low number of plasma exosomes with low levels of BTG-1 have been observed in the poor differentiation group, suggesting that plasma-exosome-derived BTG-1 might be a potential biomarker for a prognosis in patients with non-small-cell lung cancer [17][25]. Noncoding RNAs in exosomes from a variety of cells have been shown to influence metastasis via various mechanisms [18][26]. In particular, the microRNAs (miRNAs) commonly detected in exosomes are single-strand, non-encoding RNAs with the length of about 20 nucleotides, which are usually found in eukaryotic organisms [19][27]. The miRNAs can affect the stability and/or translational efficacy of their target mRNAs, consequently resulting in decreased protein translation [20][28]. More than 1000 miRNAs have been shown to regulate a lot of biological processes including proliferation, migration, and/or differentiation in cells [21][29]. Interestingly, miR590 in the exosome exerts its effects through targeting Tob1 [22][30]. miR32 might regulate the invasion of cancer stem cells, as it is upregulated in colorectal cancer tissues compared to the adjacent normal tissues [23][24][31,32]. An exosome containing miR-135a-5p could activate MMP-7 to promote liver metastasis in colorectal cancer [25][33] (Figure 1).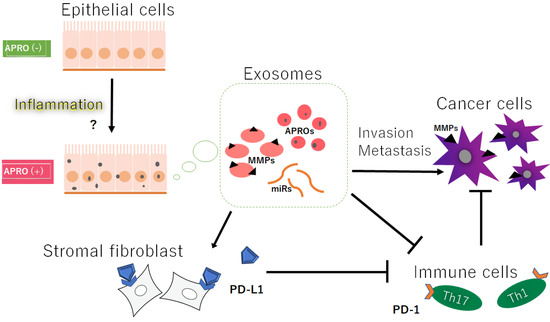
Figure 1. Hypothetical schematic image of the relationship among the APRO proteins (APROs), exosomes, immune cells, immune check point PD-L1 on stromal fibroblasts, and the cancer cells’ invasion/metastasis. Indicated molecules are examples. Arrowhead means stimulation, whereas hammerhead represents inhibition. Note that some critical pathways such as inflammation activation and/or cancer cell growth pathway have been omitted for clarity. Abbreviations: APROs—APRO family proteins; miRNAs—microRNAs; MMPs—matrix metalloproteinases; PD-L1—programmed cell-death ligand 1.
