Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ahmad Manan Mustafa Chath and Version 2 by Wendy Huang.
Metallic trace elements toxicity has been associated with a wide range of morphological abnormalities in fish, both in natural aquatic ecosystems and controlled environments. The bioaccumulation of metallic trace elements can have devastating effects on several aspects of fish health, encompassing physiological, reproductive, behavioural, and developmental functions.
- metallic trace elements
- toxicity
- growth
- fish
- ROS
- contaminated sites
- nervous system
- reproductive system
- embryonic development
1. Introduction
Recent advancements in industrialization and increased human influence on the environment have caused an exponential increment of different pollutants, such as dyes, metallic trace elements, pharmaceuticals, pesticides, fluoride, phenols, insecticides, and detergents which enter into water resources [1]. These toxicants are serious health concerns for humans and water-living organisms [2]. Similarly, surface water contamination by pesticides is also a serious health-related and environmental issue highlighted at different forums [3]. Bioaccumulation of pollutants in an aquatic ecosystem affects humans and marine life directly and indirectly through the food chain [4]. Metallic trace elements like Cd, Co, Ni, and Pb have been found to impact fishes and other aquatic organisms directly [5]. Majority of metallic trace elements also act as environmental toxins. Some of these metallic trace elements, such as Cu, Zn, Cr, Pb, Cd, Hg, and As affect the health of living beings more adversely as these could quickly transfer from one trophic level to another and hence show higher persistence in the food web [6]. Moreover, the ions of trace elements in water bodies have also become a serious concern globally, as these metallic ions have shown adverse effects on the aquatic ecosystem, and human health [7]. Therefore, simple but effective methods are required for their detection and to maintain water quality to solve water scarcity and further its reuse [8][9][8,9]. Despite being able to cause serious damage, these metals are not being identified easily due to insufficient methods and limited laboratory facilities. The present detection methods like UV–Visible spectroscopy, atomic emission spectroscopy (AAS), gas chromatography/mass spectrometries (GC/MS) are not economic and user-friendly [10]. For instance, the technological advancements have raised major concern over environmental safety, due to increasing generation of toxicants [11]. To overcome this and provide ease of analysis, with accuracy and cost effectively, “biosensor” came to existence. A biosensor has a readable biological element, responsible for providing and transforming the information that is used to detect the concentration of a particular analyte in environment. The bio-element based sensors are qualitative, quantitative, and semi-quantitative and can be used against conventional methods [12]. Biosensors possess unique features that make them more adept at measuring the level of metallic trace elements concentration on-site and therefore are advantageous in water quality control. For instance, ligand-rich membranes like tannin-reinforced 3-aminopropyltriethoxysilane crosslinked polycaprolactone (PCL) based nanofibrous membrane have shown effective and quick response to trace elements’ toxicity as compared to uncross linked membranes [13].
Figure 1 illustrates the impact of metallic trace elements on fish from different sources. These selected metals belong to the first transition series of the periodic table and are known to trigger the production of reactive oxygen species (ROS) in living systems, which contribute to their toxicity [14][15][38,39]. Exposure to sub-lethal or lethal concentrations of metallic trace elements can lead to stress in fish, which eventually accumulates in various tissues and organs such as gills, kidneys, liver, skin, muscles, etc. [16][40]. Fish have their defence mechanism to cope with the stressful conditions caused by metallic trace elements exposure by utilizing more energy from reserved carbohydrates, proteins, and lipids in their body. Metallic trace elements such as As, Cd, Cr, Cu, Fe, Hg, Ni, Pb, and Zn are active redox components that contribute to the formation of ROS, which play an essential role in certain physiological functions in fish [15][39].
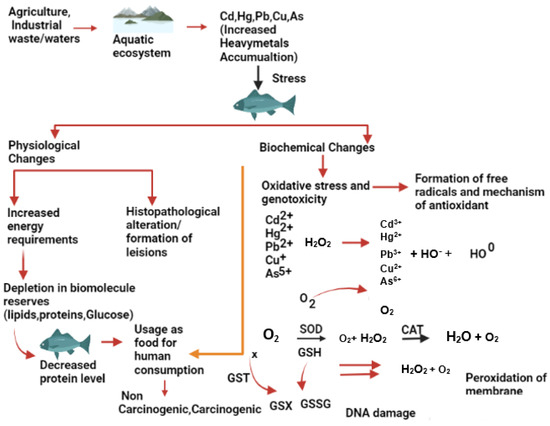
Figure 1.
Effects of metallic trace elements on the fish physiology and biochemistry.
2. Effect of Metallic Trace Elements on Fish Collected from Contaminated Sites
Estuaries are highly sensitive zones that serve as a natural conduit for transferring agricultural, industrial, and urban pollution to the sea [19][43]. Rapid industrial growth during the past century has led to an increase in industrial effluents [20][44] and anthropogenic run-off in coastal and estuarine environments [21][45]. The fate of metallic trace elements in water is mainly influenced by their initial concentration and several chemical, physical, and biological factors [22][46]. Table 1 provides details on the effects of metallic trace elements on fish collected from various contaminated sites.Table 1.
Effects of heavy metals on fish collected from different contaminated sites.
| Fish Specie | Location | Metal Detected |
Organ Affected | Effect on Fish | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metal Composition | Stage of Exposure | Exposure Duration | Effect Observed on Fish | References | |||||||
| Channa striata, Heteropnuestes fossilis |
Yamuna Barrage (India) | Cr, Ni, Pb | Kidney, gills, liver, muscle |
Ruptured veins, hemorrhages in the liver, necrotic urinary tubules. | [23][47] | ||||||
| Effect of Cadmium (Cd) | |||||||||||
| Clarias gariepinus | Abuja (Nigeria) | Pb, Cd, Cu, Zn, Cr | Liver, gill, kidney, spleen | ||||||||
| Danio rerio | Congested central veins in the liver, interstitial hemorrhages in the kidney, congested splenic vein. | 0.970 | [ | 24 | ] | Cd † | Juvenile[ | 12 h48] | |||
| Elevated immunotoxicology. | [ | 48 | ] | [ | 72 | ] | Cyprinus carpio | Slovak University of Agriculture in Nitra, University Farm Kolíňany | Cu, As, Pb, Cr, Cd, Hg | Testes | Reduced sperm DNA fragmentation, reduced motility of spermatozoa. |
| Danio rerio | 0.040 | [ | 25 | ] | [49] | ||||||
| CdCl | 2 | 0–168 hpf | 7 days | Increased rotational movement, Hyperactivity, and decreased size of otolith. | [ | 49 | |||||
| ZnSO | |||||||||||
| 4 | |||||||||||
| ·5H | |||||||||||
| 2 | |||||||||||
| O | |||||||||||
| Adult | |||||||||||
| 45 days | |||||||||||
| Significantly increased AChE activity. | |||||||||||
| [ | |||||||||||
| 72 | |||||||||||
| ] | |||||||||||
| [ | |||||||||||
| 96 | |||||||||||
| ] | |||||||||||
| Danio rerio | |||||||||||
| 1750 | |||||||||||
| ZnCl | |||||||||||
| 2 | Adult | 25 days | Significant decrease in acetylcholinesterase activity and abnormal neural signaling. | [ | 73 | ][97] | |||||
Note: † Metallic trace elements written without their respective chemical formulas were administered in their metallic forms.
4. Effect on the Reproductive System
The adverse effects of metals on the fish reproductive system are increasing every day, mainly due to increased water pollution and the usage of polluted water for fish culture. Healthy eggs and sperms are essential for the process of successful fertilization. However, the quality of eggs and sperm is affected by induced spawning, gamete storage methods, and more importantly, water pollution. The motility time of spermatozoa is very important for effective fertilization. According to the literature, sperm motility is affected by metallic trace elements. For example, although the sperm morphology of mummichog (Fundulus heteroclitus) was not affected by methylmercury (CH3Hg), it triggered a significant loss in the motility of sperms [74][75][98,99]. Lead, Cd, and Cu caused a significant decrease in the motility of European carp (Cyprinus carpio) spermatozoa [76][77][78][100,101,102]. Similarly, Cu toxicity caused adverse effects in the spermatozoa activity in C. carpio [79][103], while Sionkowski et al. [80][104] showed that the higher concentration of Cu and Pb caused reduced spermatozoa motility in grass carp (Ctenopharyngodon idella). Likewise, the effects of Zn on the sperm motility of some common carp were also explored. Metallic trace elements are also responsible for several endocrine complications among fish. For example, Cd decreased the thyroid hormone level, inhibited the estrogen receptors, and interrupted the expression of growth hormone [81][105]. On the other hand, iodine metabolism interruption by Pb was also recorded to inhibit thyroid synthesis [82][106]. Prooxidative possessions of the metal ions could also cause oxidative harm to the cell membrane. They can also induce oxidative stress in fish. Lead, Pb, and Cu can also trigger the genotoxic effects on the fish [83][84][85][107,108,109]. A tabulated review of different references is provided to show the deteriorating effects of different metals on fish’s reproductive system (Table 3).Table 3.
Effects of heavy metals on reproductive system of different fish species.
| Fish Species | Metal Concentration (mg L−1) | Metal Composition | Stage of Exposure | Exposure Duration | Effect Observed on Fish | References |
|---|---|---|---|---|---|---|
| Effect of Cadmium (Cd) | ||||||
Table 4.
Effects of heavy metals on embryonic development fish species.
| Fish Species | Metal Concentration (mg L−1) | Metal Composition | Stage of Exposure | Exposure Duration | Effect Observed on Fish | References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect of Cadmium (Cd) | |||||||||||||||||||
| Heteropneustes fossilis | 0.050 | CdCl2 | Adult | 24 h | Decreased ovulation. | [86] | |||||||||||||
| Leuciscus idus | 0.1000 | CdCl2 | [ | 110 | ] | ||||||||||||||
| Egg, sperm | 21 dpf | Reduced larval survival, growth, and delayed development. | [ | 78 | ] | [102] | Pimephales promelas | ||||||||||||
| Oryzias latipes | 0.005 | 0.0019CdCl2 | CdCl212 months | 2H2O21 days | Reduced egg production. | Embryo, larva | 20 dpf | Morphological abnormalities were observed.][73] | [51][75] | Cyprinus carpio and Capoeta | Kor River (Fars Province) | Hg, Cd, As, Pb | |||||||
| [ | 122 | ] | [ | 146 | ] | Blood cells, liver, kidney | Hyperemia, cellular degeneration, and vacuolation. | [26 | |||||||||||
| Pimephales promelas | 0.003 | Cd(NO3)2 | ] | [ | Adult50] | ||||||||||||||
| 4 days | Elevated auditory threshold. | [ | Oryzias melastigma | 50 | 0.010 | CdCl2 | 5 months][74] | 30 days | Decreased gonadal development. | [87][111] | Oreochromis niloticus | ||||||||
| Cyprinus carpio | 0.06 | Challawa River (Kano, Nigeria) | |||||||||||||||||
| CdCl | 2 | Eggs | 60 dpf | Retardation in the developmental stages of eye pigmentation and spine curvature, lack of tail formation and head. | [ | 123][147] | Pimephales promelas | 0.060Zn, Cd, Fe, Pb | Muscles | Higher bioaccumulation in muscles compared to bioaccumulation factor. | Prochilodus magdalenae | 24.90 | CdCl2 | 2 years | 7 days | Reduced fertility rate. | [88][112] | ||
| Danio rerio | 34.8 | CdCl2 | 72 hpf | 72 h | [ | CdCl227] | Adult[51] | ||||||||||||
| 21 days | Decreased vitellogenin gene expression and increased estrogen receptor beta. | [ | 51 | ] | [ | 75] | Neuromast damage, coagulated egg, increased mortality rate. | [124][148] | Clarias gariepinus | Effect of Mercury (Hg) | Lake Maryout (Egypt) | Cd, Pb, Hg, As | Gonads | The ovary exhibits lytic characteristics with oocytes at various stages, a decreased quantity of germinal cells, and an augmented interstitial space in the testes. | |||||
| Danio rerio | 0.112 | CdCl2 | [ | 28][52] | |||||||||||||||
| 0–96 hpf | 4 days | Immunotoxicity, behavioural alteration, and oxidative stress. | [ | 52 | ] | [ | 76] | ||||||||||||
| Danio rerio | 0.8018 | CdCl | 2 | 6 hpf | |||||||||||||||
| 24 h | Increased apoptotic event and induced cell death in brain of embryo. | [ | 125 | ] | [ | 149] | Auchenoglanis occidentalis | Tiga Dam (Nigeria) | Zn, Cd, Pb, Fe | Gills, liver, kidney | Lesions in the gills, liver, and kidney. | [29 | |||||||
| Effect of Mercury (Hg) | Heteropneustes fossilis | 0.050 | HgCl2 | Adult | 24 h | Increased germinal vesicle breakdown. | [86][110] | ||||||||||||
| Leuciscus idus | 0.1 | CdCl2 | ] | [ | Embryonic and larval53] | ||||||||||||||
| 21 days | Reduced embryonic survival, increased frequency of malformation, and delayed hatching. | [ | 78 | ] | [ | 102] | Hypophthalmichthys molitrix, Ctenopharyngodon idellus, Carassius auratus, | Cyprinus carpioCyprinus carpio, Silurus asotus | Yangtze River | Cd, Cr, Cu, Hg, Pb, Zn | 4.990Fish size | Positive and negative relationships were observed between fish size and metal concentration. | [ | ||||||
| Diplodus sargus | HgCl | 30 | ] | 0.0022 | 3 years[ | 12 h54] | |||||||||||||
| HgCl | 2 | Juvenile | 7 days | Increased anxiety, decreased number of optic tectum cells, and altered swim behaviour. | Decreased motility and fertility of sperms, damaged eggs. | [89][ | |||||||||||||
| Danio rerio | 0.8909 | CdCl2 | 113 | Channa striatus, Heteropneustes fossilis |
Kali River (India) | Cr, Cd, Pb, Ni | Liver, kidney, gill, muscle, brain | Decreased level of glutathione (GSH), increased oxidative stress. | [31][55] | ||||||||||
| [ | 53 | ] | [ | 77 | ] | ||||||||||||||
| ] | |||||||||||||||||||
| Embryonic and larval | 96 hpf | Increased heartbeat rate of larvae and decreased brain size. | [ | 126 | ] | [150] | Pimephales promelas | 0.720 | MeHg | Oncorhynchus mykiss | 10.00 | HgClAdult | 230 days | Decreased levels of dopamine and hyperactivity. | [54] | 3 years[ | 4 h | Reduced motility of sperm. | [90][114] |
| Leuciscus idus L. | 0.1 | CdCl2 | Embryos and newly hatched larvae | 78 | 2 h | ] | Reduced egg swelling, slowed the rate of development (especially body movements), and delayed hatching. | [127][151] | Etroplus maculates, Cirrhinus reba, and Ompok bimaculatus | Bhadra River | |||||||||
| Danio rerio | (Karnataka) |
Cu, Zn, Cd, Ni, Fe, Pb | Liver, kidney, muscle, gills |
Degeneration of the hepatocytes in liver, vacuolar degeneration in the tubular epithelium in kidney. | [32][56] | ||||||||||||||
| 10 | MeHg | Adult | 56 days | Mitochondrial dysfunction, and | oxidative phosphorylation. |
Danio rerio | 0.015 | HgCl2 | Adult | 5 days | Delayed gonadal development, imbalanced sex hormone.[ |
[91] | |||||||
| Odontesthes bonariensis | 0.00025 | 55 | ] | CdCl2 | [ | 79] | [115] | ||||||||||||
| Advanced-stage embryos and newly hatched larvae | 10 days | Decreased hatching rate and survival of embryo and larvae. | [ | 103 | ] | [127] | Oreochromis niloticus, Geophagus brasiliensis, Hoplias malabaricus, Astyanax altiparanae, Rhamdia quelen | Sao Francisco do Sul River (Brazil) | Cr, Mn, Fe, Ni, Cu, Zn, As, Se, Pb | Muscle, liver, and gonads | Metals accumulated in the gonads, liver, and muscle, with chromium levels in the muscle reaching fifty times the maximum limit set by Brazilian legislation. | [33 | |||||||
| Danio rerio | 0.720 | MeHg | ] | Adult and embryo | [57] | ||||||||||||||
| Danio rerio | 0.030 | HgCl | 30 days | 2 | AdultDecreased level of dopamine and hyperactivity. | 30 days[56] | Decreased testosterone level.[80] | [92][116] | |||||||||||
| Effect of Mercury (Hg) | Oligosarcus spp., Chyphocharax voga | Sinos River (Brazil) | Al, As, Cd, Co, Cr, Cu, Fe, Mn, Zn, Pb | Clarias gariepinus | 0.119Liver | HgCl2Detritivores species accumulated more metals than carnivorous species. | [34][58] | ||||||||||||
| Danio rerio | 0.027 | HgCl2 | 5–72 hpf | ~3 days | AdultHyperactivity causing mortality. | 30 days[57] | Disruptive effect on gamete development.[81] | [93][117] | Salminus franciscanus | Paraopeba River (Brazil) | Cu, Pb, Cd, Zn, Cr, Hg, Fe | Liver, spleen, and muscle | |||||||
| Danio rerio | 0.016 | Hepatocytes exhibited fat accumulation along with pigmented macrophages in the liver. Fibrosis was observed in the spleen, and contaminated fish showed decreased oocyte diameter and increased follicular atresia. | [ | 35 | ][59] | ||||||||||||||
| Effect of Lead (Pb) | HgCl | 2 | Adult | Pseudoplatystoma corruscans | |||||||||||||||
| Effect of Lead (Pb) | 168 hpf | Decreased hatching rate, increased mortality, increased malformation rate in larvae. | [ | 92 | ] | [116] | Paraopeba River (Brazil) | Hg, Cd, Zn, Cr, Pb | |||||||||||
| Danio rerio | 0.010 | Pb(CH3COO)2Liver, muscle, and spleen | The liver and spleen showed higher concentrations of metals compared to the muscle. Additionally, liver fibrosis was observed. | [ | 0–72 hpf36 | 3 days][60] | |||||||||||||
| 10 Gene expression changes in 89 genes associated with nervous system development. | [ | ||||||||||||||||||
| Heteropneustes fossilis | 0.050 | (Pb(NO3)2 | 58 | ] | Adult | [82] | 96 h | Increased germinal vesicle breakdown. | |||||||||||
| Cyprinus carpio | 0.00001 | HgCl2 | [ | 86 | ] | [110] | Embryo | 96 h | SOD and GPx reduced up to 85%. | [129][153] | Bryconamericus iheringii | Ilha River (Brazil) |
Al, Cd, Mn, Ni, Fe, Pb, Cr, Zn | Blood—micronucleus analysis, gills, and muscle | In rural areas, a higher frequency of micronuclei, nuclear abnormalities, and mucous cells was detected. Conversely, urban areas exhibited a lower condition factor, higher frequencies of lamellar alterations, and higher concentrations of chromium (Cr) and nickel (Ni) in muscle. | [ | |||
| Danio rerio | 0.020 | Pb(CH3 | |||||||||||||||||
| Clarias gariepinus | COO) | 2 | 140.0 | Pb(C2H3O2)2 | Adult37] | 0–144 hpf[61] | |||||||||||||
| 96 days | Reduced sperm motility. | [ | 94 | ] | [ | 118] | Prochilodus magdalenae, Pimelodus blochii | Magdalena River (Colombia) | Cd, Pb, Ni | Gills, liver, and muscle | Pimelodus Blochii showed a higher accumulation of metals, particularly an increased concentration of cadmium (Cd) in the liver. | [38 | |||||||
| Oryzias melastigma | ] | [ | 62 | ] | |||||||||||||||
| Aequidens metae, Astyanax bimaculatus | Ocoa River (Colombia) |
Hg, Cd | Blood and liver | There was a decrease in the number of erythrocytes, lymphocytes, and neutrophils, as well as a decrease in hemoglobin concentration and hematocrit percentage. | [39][63] | ||||||||||||||
3. Effect on the Nervous System
Deposition of various metallic trace elements in fish can cause serious damage to the nervous system, affecting behaviour, response to stimuli, and recognition patterns among fish [40][64]. Mercury is known to cause numerous disorders, primarily on the biochemical level in the central nervous system of fish. For example, exposure to HgCl caused a significant increase in lipid peroxidation and depletion of total lipids in the brain of catfish (Heteropneustes fossilis) [41][65]. Copper-induced morphological abrasions are evident in the sensory organs of fish [40][64]. Copper is a vital metal and a fundamental component of many enzymes, but it can be extremely toxic to fish when its concentration exceeds normal levels [42][66], especially in freshwater due to the high ionic copper content [43][67]. Increased Cu concentration in cellular membranes reduces the antioxidative capacity of lipids, causing lipid peroxidation and severe damage to cellular membranes [44][68]. As the formation of free radicals and lipid peroxidation increases, they can cause serious cellular trauma. In Cu-exposed marbled electric ray (Torpedo marmorata), ultrastructural analysis of neurons in the central nervous system showed an increased number of lipofuscin granules erosion of mitochondria [45][69] and a reduction in Golgi apparatus as well [46][70]. Long-term exposure to Pb can cause neurochemical changes in the brain of walking catfish (Clarias bathrachus). For instance, Pb increases the histamine and serotonin levels while decreasing the gamma-aminobutyric acid (GABA), monoamine oxidase (MAO), and acetylcholinesterase (AChE) contents. Furthermore, cholesterol, brain lipid, and protein contents are also decreased [47][71]. The adverse effects of different metals on the nervous system (CNS and peripheral) from various studies are compared (Table 2).Table 2.
Effects of heavy metals on nervous system of different fish species.
| Fish Species | Metal Concentration (mg L−1) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 days | |||||||||||||||||||
| Decreased axon length and decreased locomotion (speed). | |||||||||||||||||||
| [ | |||||||||||||||||||
| 59 | |||||||||||||||||||
| ] | |||||||||||||||||||
| [ | |||||||||||||||||||
| Effect of Lead (Pb) | |||||||||||||||||||
| 83 | |||||||||||||||||||
| ] | |||||||||||||||||||
| Danio rerio | 0.100 | Pb(CH3COO)2 | 2–120 hpf | ~5 days | Altered color preference (adults). | 0.050 | PbCl2 | 5 months | |||||||||||
| Danio rerio | 0.100 | Pb (C2H3 | 30 days | O2)2 | Decreased gonadal development. | [60 | Adult][84] | ||||||||||||
| [ | 87 | ] | [ | 111 | ] | 30 dpf | Distance moved by juvenile zebra fish decreased, and swimming activity alterations in larvae and juvenile fish. | [130][154] | Danio rerio | 0.207 | Pb(CH3COO)2 | 2–24 hpf | ~2 days | Decreased learning (adults). | [61][85 | ||||
| Danio rerio | ] | ||||||||||||||||||
| Effect of Copper (Cu) | 0.005 | Pb (CH3COO)2 | Adult | 144 hpf | Delayed hatching, spinal and tail deformity, pericardial edema, and yolk swelling was observed. | [131][155] | Danio rerio | 1.730 | Pb(CH3COO)2 | 0–24 hpf | 24 h | Decreased Nrxn2a gene expression. | [62][86] | ||||||
| Danio rerio | 0.040 | CuSO4 | Adult | 30 days | |||||||||||||||
| Danio rerio | 99.885 | Pb (C2H3O | Damaged structure of gonads, altered steroid hormone level. | 2)2 | [ | Adult95 | 72 hpf][119] | Deformed CNS, increased levels of Gamma-aminobutyric acid (primary inhibitory neurotransmitter). | [132][ | Effect of Copper (Cu) | |||||||||
| 156 | ] | Cyprinus carpio | 0.60 | Cu | |||||||||||||||
| Pimephales promelas | 0.075 | CuCl2 | 12 months | 21 days | Decreased abundance of post-vitellogenic follicles, increased follicular atresia. | ||||||||||||||
| Danio rerio | 1.6 | [ | Pb (NO₃)₂ | Embryo | 120 hpf | 96][120] | Spinal malformation. | [133][157] | Juvenile | 96 h | Increases in brain ROS production, lipid peroxidation, and protein oxidation. | [63][87] | |||||||
| Capoeta umbla | |||||||||||||||||||
| Daphnia magna | 1.041 | CuCl2 | Adult | 21 days | Reduced rate of reproduction. | ||||||||||||||
| Pterophyllum scalar | 20 | [ | 97 | ] | [ | 121] | PbCl2 | Embryo | 3 days | Tilt, loss of vision or the lack of effect on growth delay. | [134][158] | 3.0 | CuSO4∙5H2O | 112 ± 5 g | 96 h | Induce astroglial response accompanied by modulations of NF-kB and PARP-1 expression. | [ | ||
| Poecilia reticulata | 45 | 64 | CuO | Adult | |||||||||||||||
| Effect of Copper (Cu) | Larvae | ] | [ | 88] | |||||||||||||||
| 96 h | Decreased reproduction success. | [ | 98 | ] | [122] | Danio rerio | 0.100 | ||||||||||||
| Poecilia reticulate | CuSO | 4 | 0.026∙5H | CuSO4 5H2O | |||||||||||||||
| Gobiocypris rarus | 40.00 | NaAsO2 | 3 months | 96 days | Accumulation in testis. | ||||||||||||||
| Danio rerio | 0.016 | HgCl2 | Adult | 2 hpf | T3 and T4 content in larvae increased. | [128][152] | 2O | Adult | 10 days | Negatively affect the associative learning capabilities. | [65][89] | ||||||||
| 2.5–3 months | 56 days | Gonadosomatic index, offspring production decreased. | [ | 99 | ] | [123] | |||||||||||||
| Leuciscus idus | 0.100 | CuSO4·5H2O | Egg, sperm | 21 dpf | Reduced larval survival, growth, and delayed development. | [78][102] | Oreochromis niloticus | 120 | CuSO4∙5H2O | Adult | |||||||||
| Oryzias latipes | 96 h | 0.0185 | CuCl | Loss of balance and exhaustion. | 2 H2O | [66][90] | |||||||||||||
| Effect of Arsenic | Embryo, larva | 20 dpf | Percentage of deformed larvae significantly increased. | [122][146] | Effect of Arsenic (As) | ||||||||||||||
| Poecilia reticulata | 1.50 | CuSO4·5H2 | |||||||||||||||||
| O | [ | Embryo | 15 days | Abnormalities in blastodisc to middle-eyed stages of development. | 100 | ][124] | [135][159] | Danio rerio | 15 | Na2HAsO4 | Adult | ||||||||
| Daphnia magna | 0.049 | 96 h | NaAsO2 | Alteration in behaviour and ectonucleotidase activities. | Adult | [67] | 48 h[91] | ||||||||||||
| Stable reproduction rate. | [ | ||||||||||||||||||
| Danio rerio | 0.018 | CuSO4 | 101 | ] | [ | 125] | 72 hpf | 72 h | Neuromast damage, coagulated egg, increased mortality rate. | [124][148] | Danio rerio | 0.050 | As2O3 | Juvenile | 96 h | ||||
| Gambusia affinis | 0.075 | NaAsO | |||||||||||||||||
| Leuciscus idus | 0.10 | CuSO4 | Antagonistic effects on brain. | 2 | ·5H2OJuvenile | [68 | 30 days][92] | ||||||||||||
| Lower gonadal-somatic indices. | Embryo and larval | [ | 102 | ] | [ | 126] | 21 days | Reduced embryonic survival, increased frequency of malformation. | [78][102] | Danio rerio | |||||||||
| Leuciscus idus L. | 0.500 | As+ | Larvae, juvenile and adult | 96 h | |||||||||||||||
| Effect of Zinc (Zn) | Alteration in motor function | (embryo-adult), effects on associative learning. | [69][93] | ||||||||||||||||
| 0.10 | CuSO4 | Embryos and newly hatched larvae | 2 h | Reduced egg swelling slowed the rate of development (especially body movements) and delayed hatching. | [127][151] | Clarias batrachus | 20 | As2O3 | |||||||||||
| Odontesthes bonariensis | 0.021 | ZnSO | Adult | 496 h | Increased body discoloration, excessive mucous secretion, loosening of the skin, and complete loss of skin (head region and fins). | 7H2O[70][94] | |||||||||||||
| Adult | 10 days | Reduced embryo and larval survivability. | [ | 103 | |||||||||||||||
| Odontesthes bonariensis | 0.00025 | ] | CuSO4 | Advanced-stage embryos and newly hatched larvae | [ | 10 days127] | Decreased hatching rate and survival of embryo and larvae. | [103][127] | Effect of Zinc (Zn) | ||||||||||
| Danio rerio | 500 | Zn | Adult | ||||||||||||||||
| 4 days | Majority of eggs were dead, larger hatching time. | [ | 104 | ] | |||||||||||||||
| Carassius auratus | 1 | [ | Cu2− | Embryo | 24 h post-hatching | Scoliosis and tail curvatures.128] | [136][160] | Anguilla anguilla | 0.12 | Zn | |||||||||
| Effect of Arsenic (As) | Juvenile | 28 days | Cholinergic neurotoxicity did not occurr, only liver GST increased significantly. | [71][95] | |||||||||||||||
| Clarias magur | 300 | Zn(CH3COO)2 | Mature | 60 days | The highest GSI and fecundity. | [105][129] | Leporinus obtusidens | 4.57 | |||||||||||
| Oryzias melastigma | 0.010 | ZnSO4·7H2O | Adult | 30 | Irregular oocytes, partly adhesion, empty follicle, and increased follicular atresia, loose follicular lining. | [87][111] | |||||||||||||
5. Effect on Embryonic Development
The influence of water-borne metals can disrupt the embryonic development of spawners. [106][130] found elevated levels of Cd, Zn, and Pb in the female gonads of stone loach when exposed to toxic concentrations of these metals. Ellenberger et al. [107][131] investigated the levels of Cu in the reproductive organs of European perch (Perca fluviatilis) exposed to Cu-polluted ponds. White suckers in polluted lakes exhibited higher amounts of Cu and Zn in their testicles and female gonads compared to fish in uncontaminated water [108][109][132,133]. Common carp exposed to Cu, Cd, and Pb showed decreased egg swellings in a concentration-dependent manner, contrasting with about 40% expansion in egg width observed in the untreated groups [110][134]. Copper and Cd accumulation in the gonads of Mozambique tilapia (Orechromis mossambicus) was found to be elevated when fish were kept in metal-polluted water, and blue tilapia (Oreochromis aureus) exposed to Cd and Pb for seven days showed metal accumulation in the testicles and female gonads, particularly Cd levels in the ovaries [111][135]. Metal exposure to spawners can result in the deposition of metallic trace elements accumulated in eggs and sperm, severely affecting the survival of fertilized eggs and the embryonic development of fish [79][103]. Metals can also influence the physical characteristics of an egg’s outer surface. Benoit and Holcombe [112][136] observed that eggs of Zn-exposed fathead minnow (Pimephales promelas) became sticky and more prone to breakage soon after egg laying. Fathead minnow embryos rapidly absorb Hg from surrounding water sources, with concentrations in juveniles increasing to 2.80 µg per gram humid mass after four days of exposure to 25 µg per cubic decimeter of methylmercury [113][137]. Chromium was found to accumulate in the outer protective coatings of Cyprinus carpio eggs at pH 6.3 [114][138]. Copper can alter selective membrane permeability, disrupting cation trade between the liquid in the yolk membrane and the outside water [115][139]. During the early development of fish eggs in a toxic (metallic trace elements) environment, the outer protective coating of the egg blocks most of the metal concentration, but a significant toxic amount still enters the fluid inside the egg membranes, while only a small amount infiltrates the embryo [116][140]. Beattie and Pascoe [117][141] found that eggs of Atlantic salmon (Salmo salar) exposed to 10 mg per litre of Cd at 22 h old retained 98% of the metal in the outermost membrane. Similarly, the outer membrane of Japanese rice fish (Oryzias latipes) eggs retained 94.4% of Cd [118][142]. In Zn-treated Atlantic herring (Clupea harengus) eggs, 30% to 50% of Zn accumulated in the outermost membrane, while the rest accumulated primarily in the yolk sac and in lower quantities in the embryos. However, even a small amount of metals penetrating the egg can significantly influence fish embryonic growth [117][119][141,143]. Devlin [113][137]| Danio rerio | ||||||
| 360.32 | ||||||
| NaAsO | ||||||
| 2 | ||||||
| Adult | ||||||
| 120 h | ||||||
| Tail bud deformation in embryo. | ||||||
| [ | ||||||
| 137 | ||||||
| ] | ||||||
| [ | ||||||
| 161 | ||||||
| ] | ||||||
| Danio rerio | ||||||
| 0.5 | ||||||
| NaAsO | 2 | Adult | 120 hpf | No effect on mortality and developmental deformations. | [ | 138][162] |
| Labeo rohita | 198.18 | NaAsO2 | Adult | 120 hpf | Reduced survival rate with abnormal development. | [139][163] |
| Danio rerio | 0.5 | NaAsO2 | Embryo | 14 dpf | Thinning of the retinal pigmented epithelium (RPE) layer in embryos. | [140][164] |
| Effect of Zinc (Zn) | ||||||
| Odontesthes bonariensis | 0.021 | ZnSO4 7H2O | Hatchling | 10 days | Cumulative embryo survival was significantly reduced. | [103][127] |
| Pagrus major | 2.5 | ZnCl2 | 2 years | 10 days | Low hatching rate, high mortality, abnormal pigmentation, hooked tail, spinal deformity, pericardial edema, and visceral hemorrhage. | [141][165] |
| Melanotaenia fluviatilis | 33.3 | Zn | Embryo | 2 h | Spinal deformities. | [142][166] |
