Biomaterials are at the forefront of the future, finding a variety of applications in the biomedical field, especially in wound healing, thanks to their biocompatible and biodegradable properties. Wounds spontaneously try to heal through a series of interconnected processes involving several initiators and mediators such as cytokines, macrophages, and fibroblasts. The combination of biopolymers with wound healing properties may provide opportunities to synthesize matrices that stimulate and trigger target cell responses crucial to the healing process.
- wound healing
- wound management
- dressings
- biopolymers
- natural polymers
- synthetic polymers
- hydrogels
- electrospinning
- 3D printing
1. Introduction
1.1. Physiological Native Skin
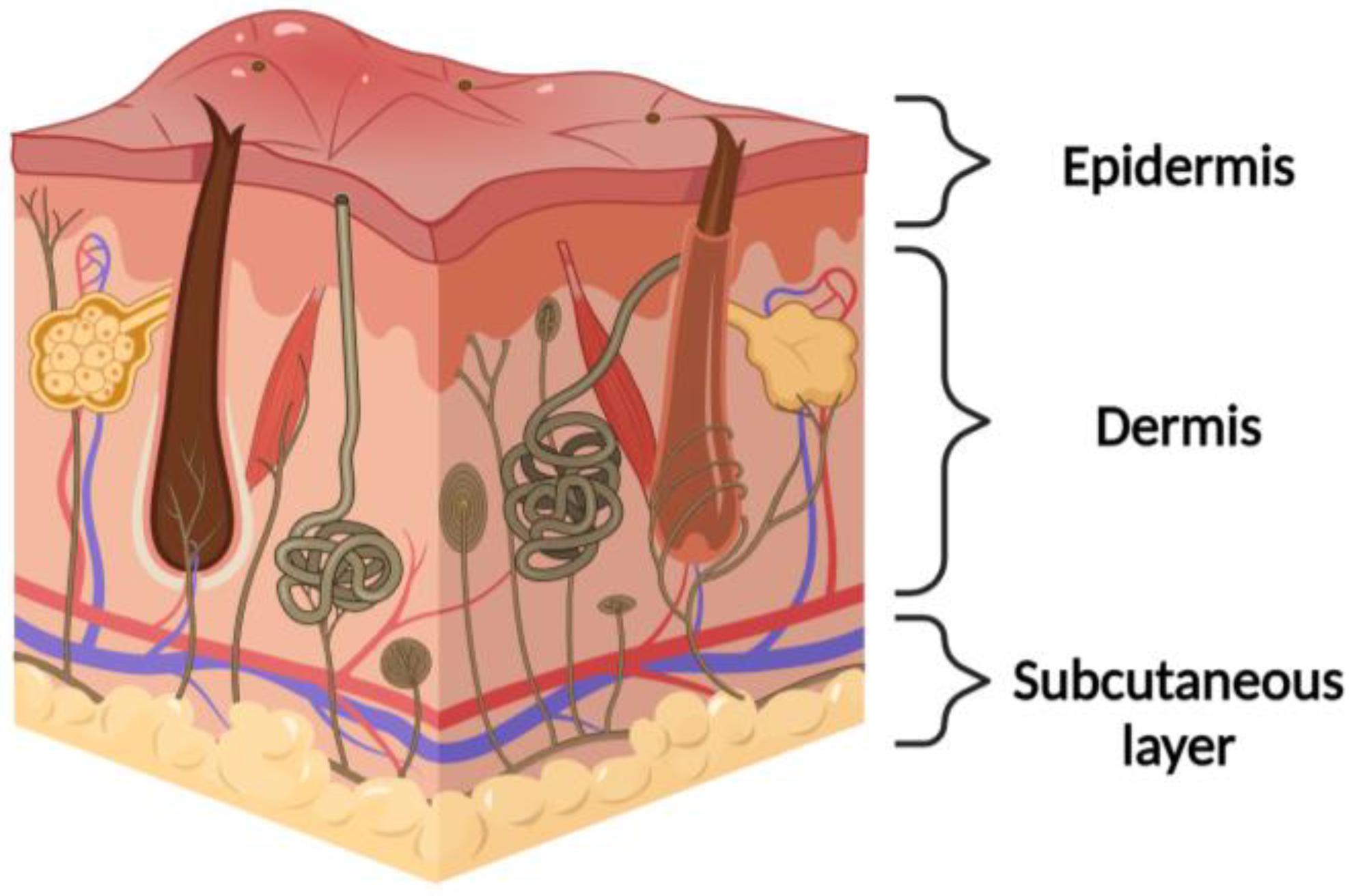
1.2. Wound Healing Process
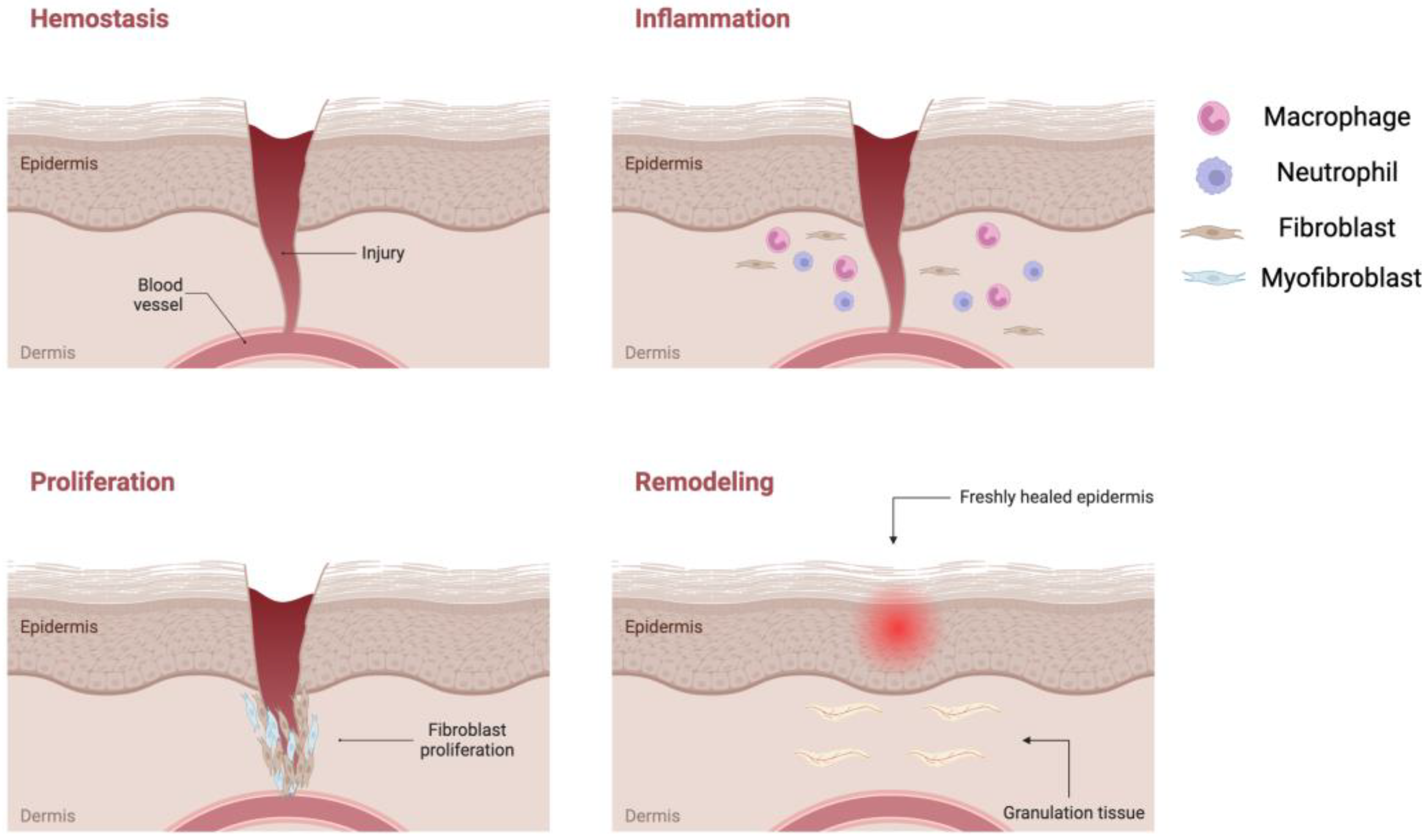
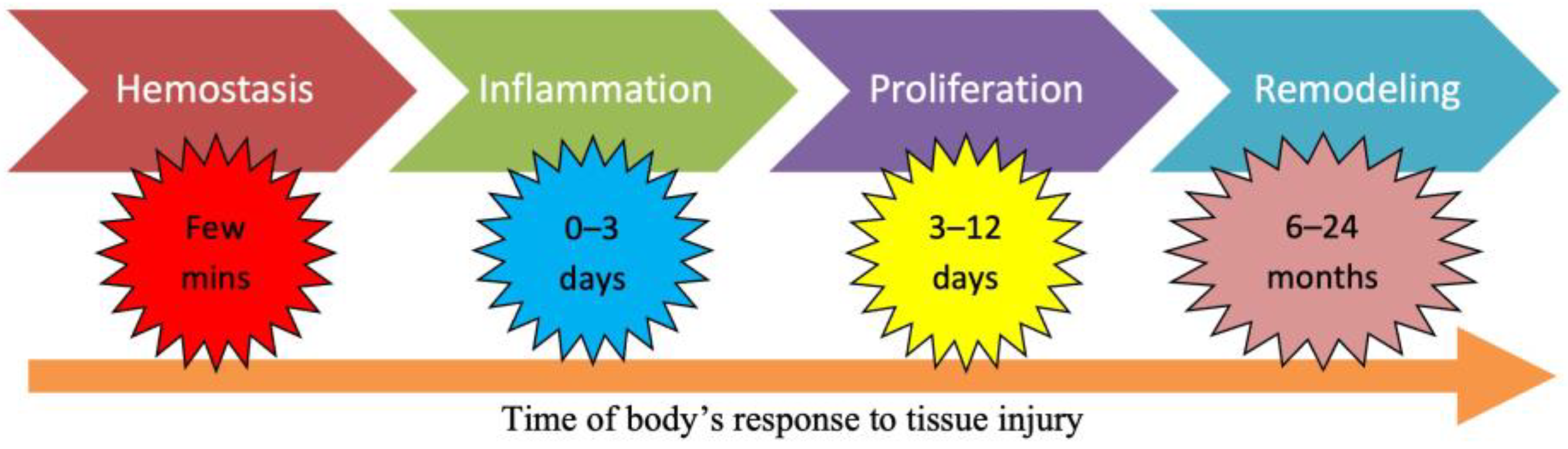
1.3. Wound Management
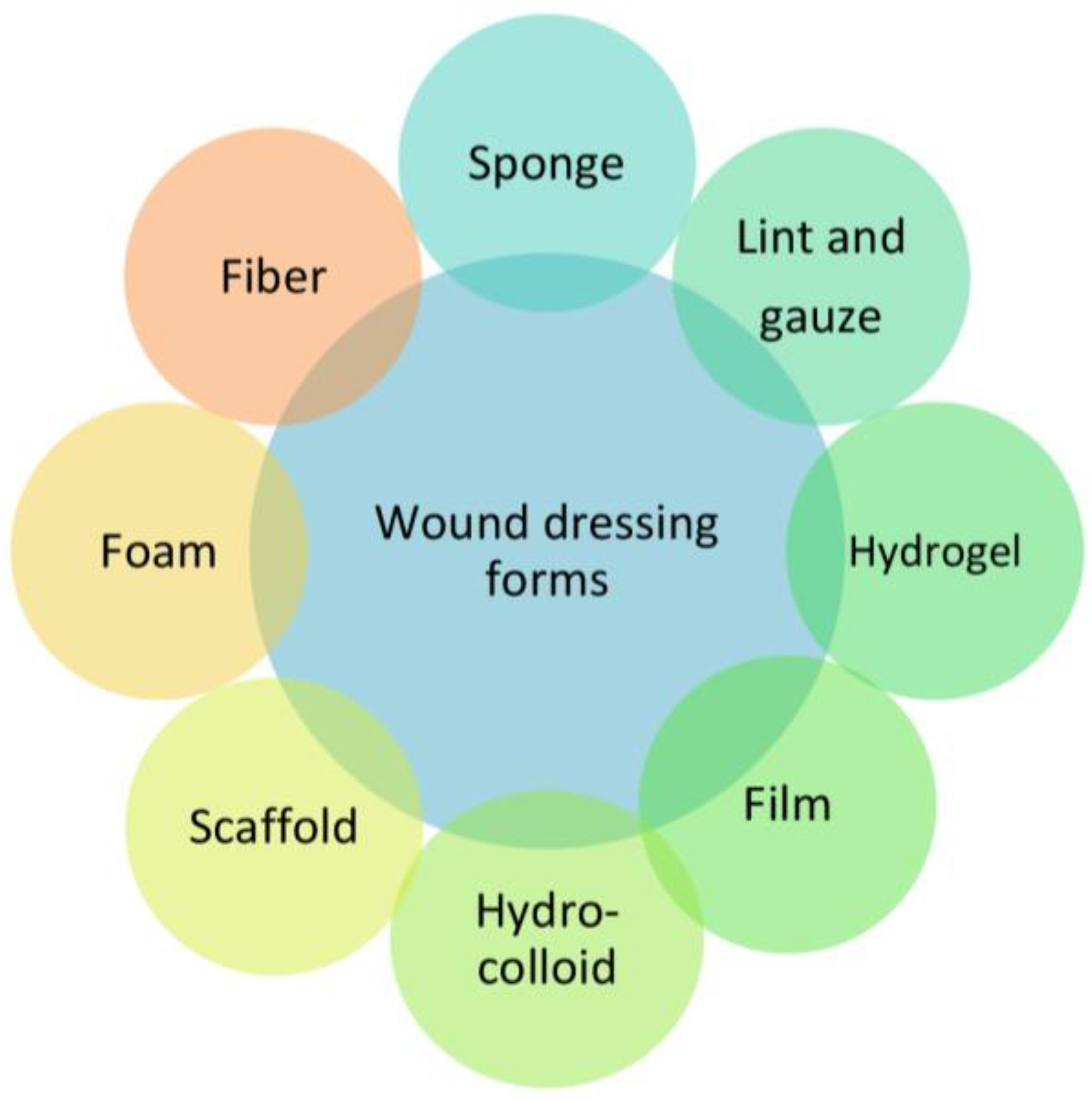
2. Biomaterial-Based Dressings
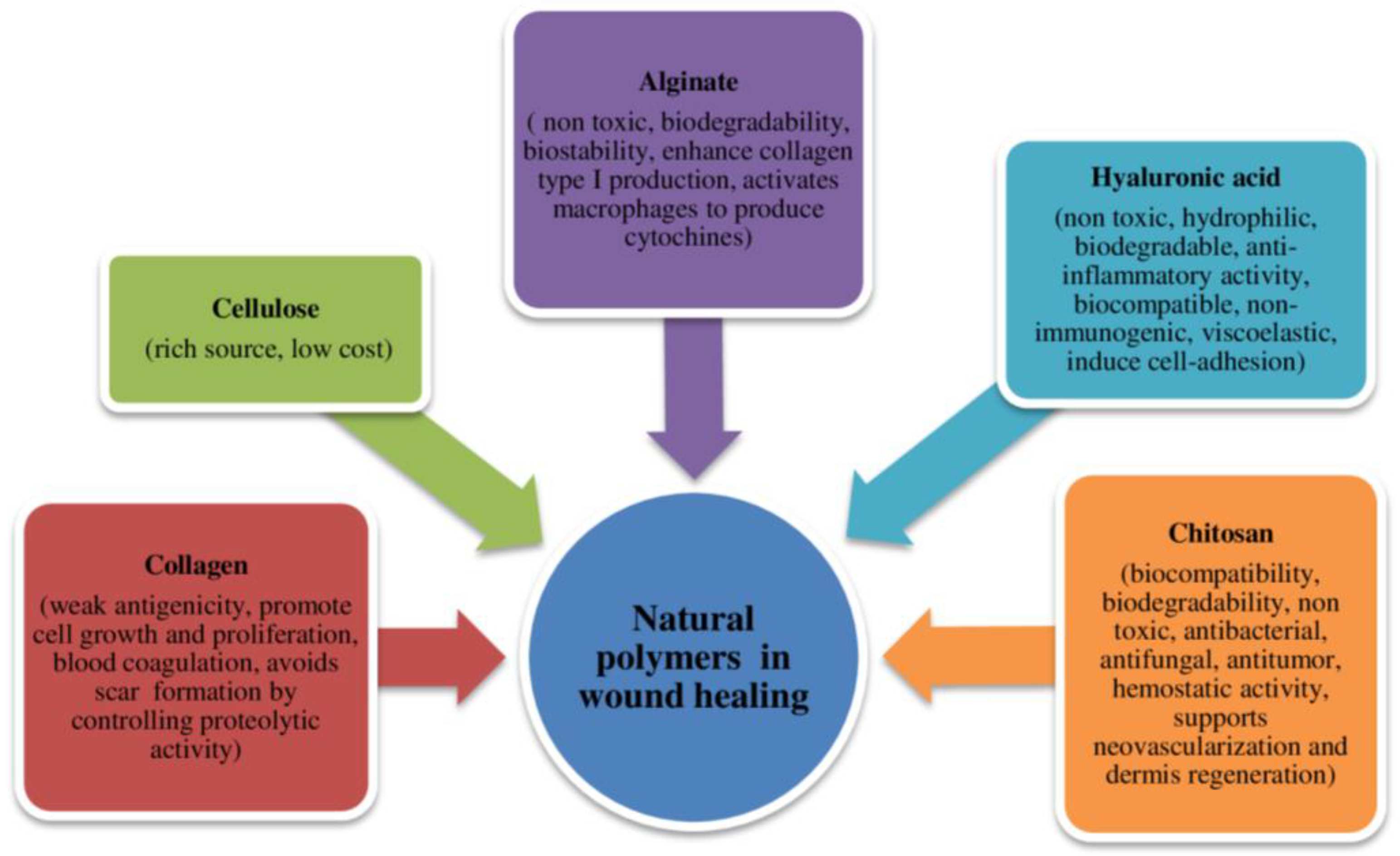
2.1. Natural Polymers
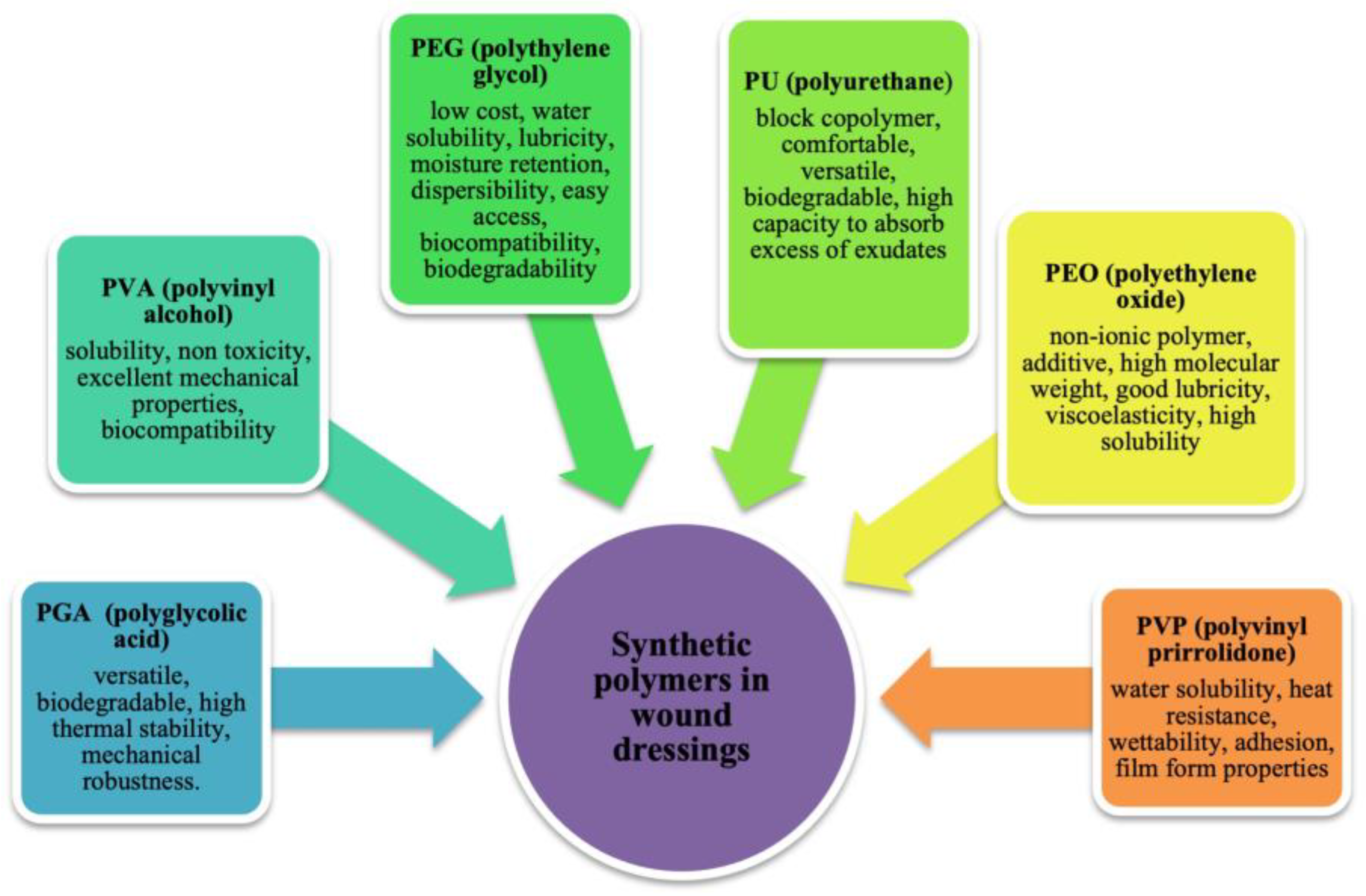
2.2. Synthetic Polymers
3. Classification of the Dressings by Physical Form
3.1. Bandages
3.2. Lyophilized Wafers
3.3. Hydrogels
3.4. Films
3.5. Patches
3.6. Scaffolds
3.7. Hydrocolloids
3.8. Foam Dressings
|
Dressings Material Form |
Materials/Polymers |
Bioactives |
Results |
Limitations |
References |
|---|---|---|---|---|---|
|
Bandages |
Cotton wool; gauze cotton with polymers as PCL |
Antibiotics and anti-inflammatory agents |
Primary and secondary dressings |
Discomfort during dressing changes, poor adhesive properties, and low drainage level for the wound |
[55] |
|
Lyophilized wafers |
Synthetic polymers: PU |
Antibiotics and growth factors |
Adhesive dressings |
Quite fragile, difficulty in application, lack of flexibility |
[42] |
|
Hydrogels |
Natural and synthetic polymers capable of creating tridimensional network: PEG, chitosan, PVA, PVP |
Antimicrobial agents and several actives |
Secondary dressings in the form of insoluble aqueous gels facilitated autolytic debridement, protecting against desiccation and creating an environment conducive to effective wound healing |
Limited absorption capacity, need to be reapplied relatively frequently, low mechanical strength |
|
|
Films |
A translucent, typically PU, thin film |
Antimicrobial agents |
Moist environment. Waterproof and transparent Semi-permeable to oxygen and water vapor while effectively blocking liquids and bacterial contamination, eliminating the need for an additional dressing |
Insufficient mechanical strength, removal can be painful, limited absorption of odor and exudate |
|
|
Patches |
Combination of biomaterials: chitosan, cellulose, HA, PVA, PCL |
Antiseptics such as AgNPs and other agents as polyhexamethylene biguanide, gold NPs, bacitracin, and metronidazole. Antioxidant agents isolated from plants such as Bletilla striata, Calendula officinalis |
Possessing therapeutic attributes such as adhesion, absorption, mechanical support, and robustness within a multi-layered structure |
Can be occlusive thus trapping moisture against the wound, limited exudate management capabilities, allergic reactions to the adhesive used in patches |
[48] |
|
Scaffolds |
Natural and synthetic polymers such as chitosan, PVA, and surfactants. |
Active drugs such as anti-inflammatories, antibiotics, and antiseptics. |
Primary and secondary dressings designed to replicate the characteristics of the skin, effectively acting as skin mimics or pseudo-skin |
Difficulty in application, can provide surfaces where bacteria can colonize, increasing the risk of infection |
|
|
Hydrocolloids |
Gel with agents such as elastomers, Gelatine, pectin, and adhesive elements |
Local anesthetics as lidocaine |
Occlusive dressings. Used in dried, black eschars and for grade 1 and 2 pressure ulcers and moisture lesions |
Do not absorb blood or bacterial infection, do not promote autolytic debridement, difficulty in removal |
[52] |
|
Foam dressing |
Hydrophilic PU |
Antimicrobials and other actives to form a barrier from microorganisms |
Utilized for wounds with moderate to high drainage levels, these dressings are exceptionally absorbent, offering cushioning, protection, and conformability to various body contours. Their absorbent nature reduces the need for frequent replacement, while their easy removal adds to their convenience |
Limited adhesion to dry wound surfaces, might saturate quickly if the wound has very high exudate levels, skin irritation or allergic reactions to the adhesive or foam material used in these dressings |
|
|
Sponges |
Hydrophilic natural and synthetic polymers such as cellulose, collagen, sodium alginate, PVA or PEG |
Antimicrobial or hemostatic bioactive molecules |
Used to absorb exudate or blood and to clean the wound |
Poor mechanical property, variable absorption capacity, “macrosponge” formation, do not maintain a controlled-moisture environment |
[76] |
|
Membranes |
Chitosan, alginate, PVA |
Antimicrobial or growth factors encapsulated |
Used for non-adherent wound, secondary damage, and injury without scarless wound healing |
Limited adsorption, might not conform well to wounds with deep crevices or irregular shapes, might not make full contact with the wound bed |
|
|
Fibers |
Cellulose |
Antibacterial agents |
Tissues engineered |
Poor mechanical properties, easy to saturate thus requiring frequent dressing changes, variable absorption capacity |
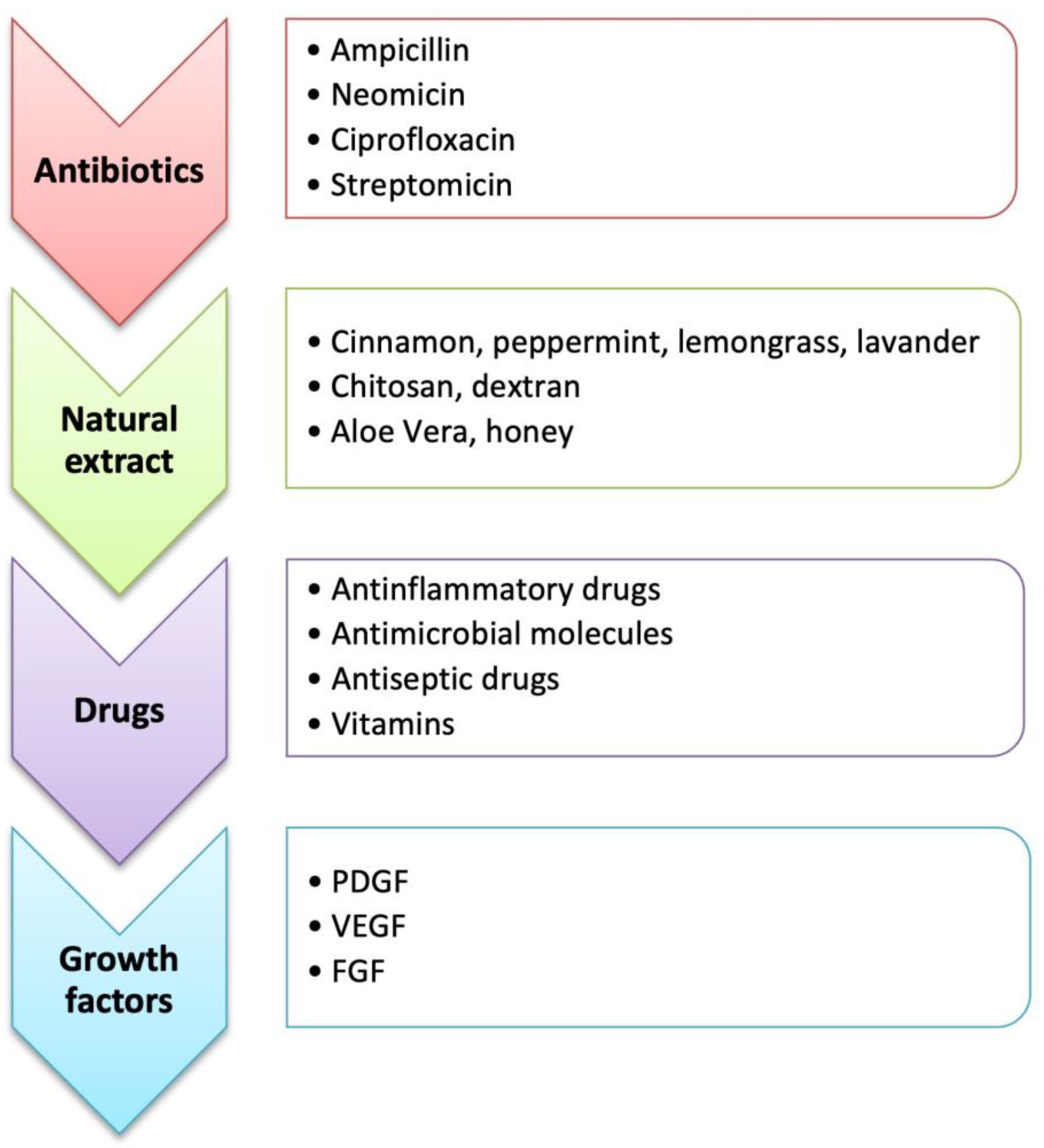
4. Bioactive Molecules
5. Methods of Preparation for Wound Dressings
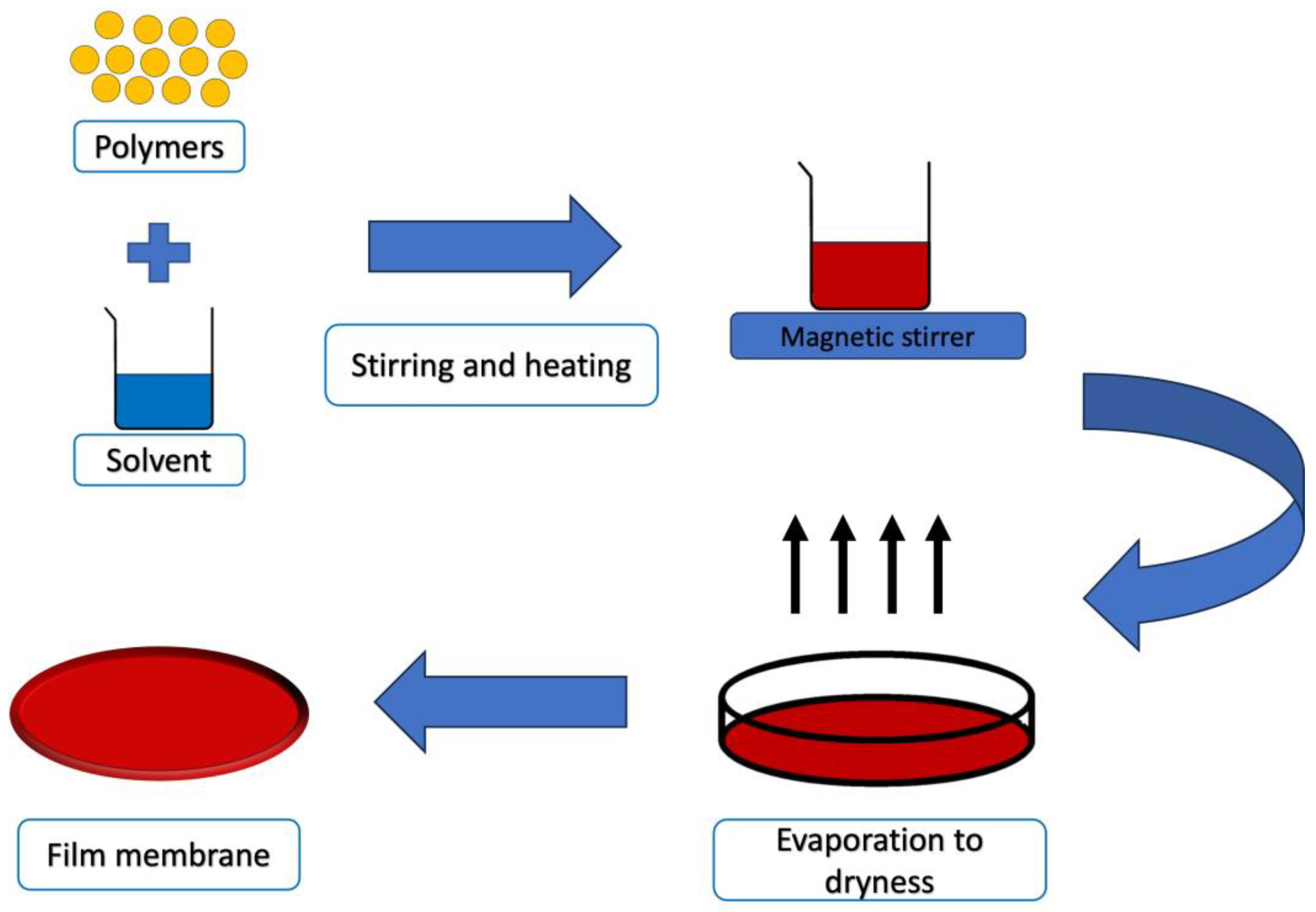
5.1. Solvent Casting Technique
5.2. Electrospinning
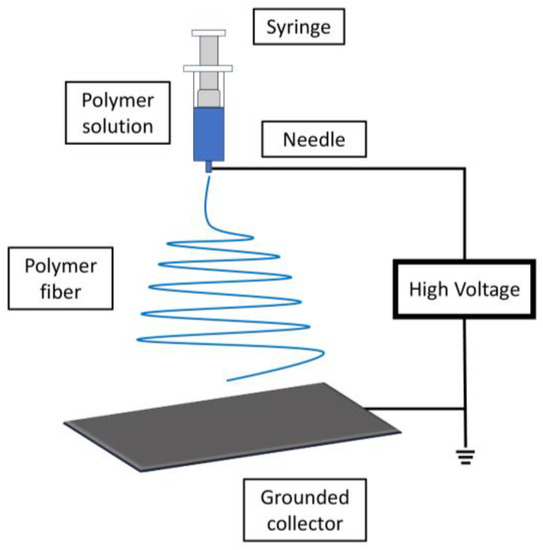
5.3. Melt-Blowing
5.4. Thermal Annealing
5.5. 3D Printing Technology
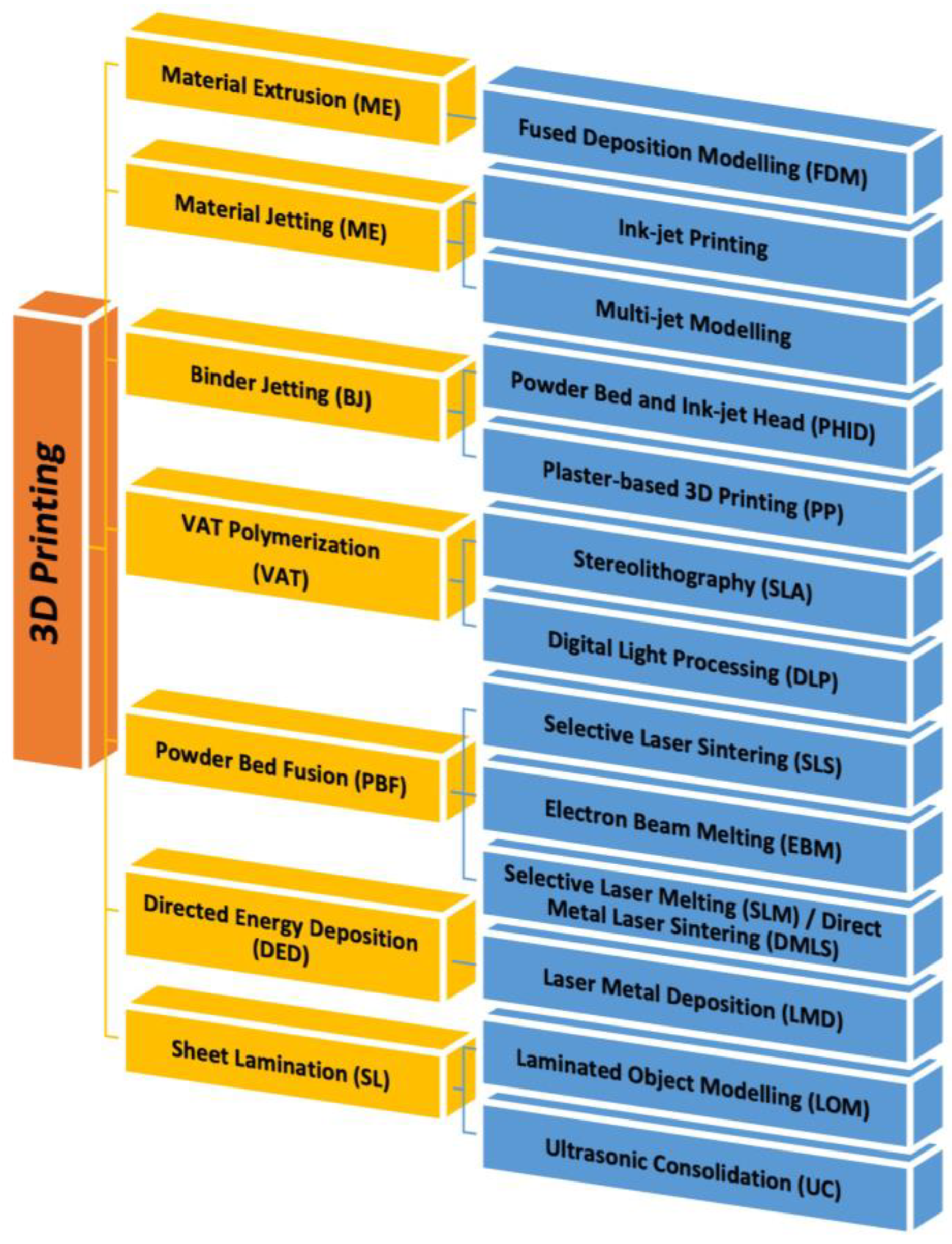
3D Bioprinting
References
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230.
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Control. Release 2015, 219, 119–128.
- Zhong, S.; Zhang, Y.; Lim, C. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525.
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.W. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Healthc. Mater. 2019, 8, 1801019.
- Joodaki, H.; Panzer, M.B. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 323–343.
- Grover, S.; Ranyal, R.K.; Bedi, M.K. A cross section of skin diseases in rural Allahabad. Indian J. Dermatol. 2008, 53, 179.
- Mostafa, W.Z.; Hegazy, R.A. Vitamin D and the skin: Focus on a complex relationship: A review. J. Adv. Res. 2015, 6, 793–804.
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735.
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289.
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700.
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321.
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162.
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525.
- Solarte David, V.A.; Güiza-Argüello, V.R.; Arango-Rodríguez, M.L.; Sossa, C.L.; Becerra-Bayona, S.M. Decellularized tissues for wound healing: Towards closing the gap between scaffold design and effective extracellular matrix remodeling. Front. Bioeng. Biotechnol. 2022, 10, 821852.
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive glasses: A promising therapeutic ion release strategy for enhancing wound healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430.
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 74, 103564.
- Stramer, B.M.; Mori, R.; Martin, P. The inflammation–fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Investig. Dermatol. 2007, 127, 1009–1017.
- Aisa, J.; Parlier, M. Local wound management: A review of modern techniques and products. Vet. Dermatol. 2022, 33, 463–478.
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1–S28.
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169.
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923.
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern wound dressings: Hydrogel dressings. Biomedicines 2021, 9, 1235.
- Weller, C.D.; Team, V.; Sussman, G. First-line interactive wound dressing update: A comprehensive review of the evidence. Front. Pharmacol. 2020, 11, 155.
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193.
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166.
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290.
- Wang, M.; Huang, X.; Zheng, H.; Tang, Y.; Zeng, K.; Shao, L.; Li, L. Nanomaterials applied in wound healing: Mechanisms, limitations and perspectives. J. Control. Release 2021, 337, 236–247.
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 2020, 8, 182.
- Heyer, K.; Augustin, M.; Protz, K.; Herberger, K.; Spehr, C.; Rustenbach, S. Effectiveness of advanced versus conventional wound dressings on healing of chronic wounds: Systematic review and meta-analysis. Dermatology 2013, 226, 172–184.
- Ongarora, B.G. Recent technological advances in the management of chronic wounds: A literature review. Health Sci. Rep. 2022, 5, e641.
- Dumville, J.C.; Gray, T.A.; Walter, C.J.; Sharp, C.A.; Page, T.; Macefield, R.; Blencowe, N.; Milne, T.K.; Reeves, B.C.; Blazeby, J. Dressings for the prevention of surgical site infection. Cochrane Database Syst. Rev. 2016, 12, CD003091.
- Enoch, S.; Grey, J.E.; Harding, K.G. Non-surgical and drug treatments. BMJ 2006, 332, 900–903.
- Agrawal, P.; Soni, S.; Mittal, G.; Bhatnagar, A. Role of polymeric biomaterials as wound healing agents. Int. J. Low. Extrem. Wounds 2014, 13, 180–190.
- Gardikiotis, I.; Cojocaru, F.-D.; Mihai, C.-T.; Balan, V.; Dodi, G. Borrowing the Features of Biopolymers for Emerging Wound Healing Dressings: A Review. Int. J. Mol. Sci. 2022, 23, 8778.
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55.
- Moholkar, D.N.; Sadalage, P.S.; Peixoto, D.; Paiva-Santos, A.C.; Pawar, K.D. Recent advances in biopolymer-based formulations for wound healing applications. Eur. Polym. J. 2021, 160, 110784.
- Qiu, L.Y.; Bae, Y.H. Polymer architecture and drug delivery. Pharm. Res. 2006, 23, 1–30.
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105.
- Williams, D.F. Biocompatibility pathways and mechanisms for bioactive materials: The bioactivity zone. Bioact. Mater. 2022, 10, 306–322.
- Mndlovu, H.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Marimuthu, T.; Kondiah, P.P.; Pillay, V. Bioplatform fabrication approaches affecting chitosan-based interpolymer complex properties and performance as wound dressings. Molecules 2020, 25, 222.
- Falabella, A.F. Debridement and wound bed preparation. Dermatol. Ther. 2006, 19, 317–325.
- Tabassum, A.; Furtado, S.C.; Bharath, S. Development of antimicrobial colloidal silver incorporated lyophilized biopolymer wafers for wound care. Wound Med. 2018, 21, 1–7.
- Kim, M.H.; Park, H.; Nam, H.C.; Park, S.R.; Jung, J.-Y.; Park, W.H. Injectable methylcellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr. Polym. 2018, 181, 579–586.
- Xie, Z.; Aphale, N.V.; Kadapure, T.D.; Wadajkar, A.S.; Orr, S.; Gyawali, D.; Qian, G.; Nguyen, K.T.; Yang, J. Design of antimicrobial peptides conjugated biodegradable citric acid derived hydrogels for wound healing. J. Biomed. Mater. Res. Part A 2015, 103, 3907–3918.
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780.
- Arroyo, A.A.; Casanova, P.L.; Soriano, J.V.; Torra i Bou, J.E. Open-label clinical trial comparing the clinical and economic effectiveness of using a polyurethane film surgical dressing with gauze surgical dressings in the care of post-operative surgical wounds. Int. Wound J. 2015, 12, 285–292.
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917.
- Pillai, M.M.; Dandia, H.; Checker, R.; Rokade, S.; Sharma, D.; Tayalia, P. Novel combination of bioactive agents in bilayered dermal patches provides superior wound healing. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102495.
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.-Y.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-printed bioinks for skin regeneration and wound healing: A systematic review. Polymers 2020, 12, 1782.
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543.
- Lam, C.X.F.; Mo, X.; Teoh, S.-H.; Hutmacher, D. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. C 2002, 20, 49–56.
- Takeuchi, T.; Ito, M.; Yamaguchi, S.; Watanabe, S.; Honda, M.; Imahashi, T.; Yamada, T.; Kokubo, T. Hydrocolloid dressing improves wound healing by increasing M2 macrophage polarization in mice with diabetes. Nagoya J. Med. Sci. 2020, 82, 487.
- Pott, F.S.; Meier, M.J.; Stocco, J.G.D.; Crozeta, K.; Ribas, J.D. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: A systematic review and meta-analysis. Rev. Lat.-Am. Enferm. 2014, 22, 511–520.
- Priddy-Arrington, T.R.; Ward, M.S.; Edwards, R.E.; Caldorera-Moore, M.E. Proactive biomaterials for chronic wound management and treatment. Curr. Opin. Biomed. Eng. 2021, 20, 100327.
- Parkale, R.; Pulugu, P.; Kumar, P. Nanomaterials decoration on commercial cotton bandages for pain and infection management. arXiv 2021, arXiv:2105.10273.
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452.
- Jeong, J.-O.; Park, J.-S.; Kim, E.J.; Jeong, S.-I.; Lee, J.Y.; Lim, Y.-M. Preparation of radiation cross-linked poly (Acrylic acid) hydrogel containing metronidazole with enhanced antibacterial activity. Int. J. Mol. Sci. 2019, 21, 187.
- Shin, J.Y.; Lee, D.Y.; Kim, B.Y.; Yoon, J.I. Effect of polyethylene glycol molecular weight on cell growth behavior of polyvinyl alcohol/carboxymethyl cellulose/polyethylene glycol hydrogel. J. Appl. Polym. Sci. 2020, 137, 49568.
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.-I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90.
- Hemmingsen, L.M.; Julin, K.; Ahsan, L.; Basnet, P.; Johannessen, M.; Škalko-Basnet, N. Chitosomes-in-chitosan hydrogel for acute skin injuries: Prevention and infection control. Mar. Drugs 2021, 19, 269.
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of alginate-based hydrogels in hemostasis. Gels 2022, 8, 109.
- Da Silva, L.; Cerqueira, M.; Correlo, V.; Reis, R.; Marques, A. Engineered hydrogel-based matrices for skin wound healing. In Wound Healing Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–250.
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023.
- Kenawy, E.-R.S.; Kamoun, E.A.; Ghaly, Z.S.; Shokr, A.-b.M.; El-Meligy, M.A.; Mahmoud, Y.A.-G. Novel physically cross-linked curcumin-loaded PVA/Aloe vera hydrogel membranes for acceleration of topical wound healing: In vitro and in vivo experiments. Arab. J. Sci. Eng. 2023, 48, 497–514.
- Khan, M.I.; Paul, P.; Behera, S.K.; Jena, B.; Tripathy, S.K.; Lundborg, C.S.; Mishra, A. To decipher the antibacterial mechanism and promotion of wound healing activity by hydrogels embedded with biogenic Ag@ ZnO core-shell nanocomposites. Chem. Eng. J. 2021, 417, 128025.
- Capanema, N.S.; Mansur, A.A.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234.
- Hosseini, M.; Razavi, S.; Mousavi, M. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009, 33, 727–743.
- Buanz, A.B.; Belaunde, C.C.; Soutari, N.; Tuleu, C.; Gul, M.O.; Gaisford, S. Ink-jet printing versus solvent casting to prepare oral films: Effect on mechanical properties and physical stability. Int. J. Pharm. 2015, 494, 611–618.
- Panayi, A.C.; Haug, V.; Liu, Q.; Wu, M.; Karvar, M.; Aoki, S.; Ma, C.; Hamaguchi, R.; Endo, Y.; Orgill, D.P. Novel application of autologous micrografts in a collagen-glycosaminoglycan scaffold for diabetic wound healing. Biomed. Mater. 2021, 16, 035032.
- Diaz-Gomez, L.; Gonzalez-Prada, I.; Millan, R.; Da Silva-Candal, A.; Bugallo-Casal, A.; Campos, F.; Concheiro, A.; Alvarez-Lorenzo, C. 3D printed carboxymethyl cellulose scaffolds for autologous growth factors delivery in wound healing. Carbohydr. Polym. 2022, 278, 118924.
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37.
- Mobaraki, M.; Bizari, D.; Soltani, M.; Khshmohabat, H.; Raahemifar, K.; Akbarzade Amirdehi, M. The effects of curcumin nanoparticles incorporated into collagen-alginate scaffold on wound healing of skin tissue in trauma patients. Polymers 2021, 13, 4291.
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272.
- Song, E.-H.; Jeong, S.-H.; Park, J.-U.; Kim, S.; Kim, H.-E.; Song, J. Polyurethane-silica hybrid foams from a one-step foaming reaction, coupled with a sol-gel process, for enhanced wound healing. Mater. Sci. Eng. C 2017, 79, 866–874.
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J. Antimicrobial lignin-based Polyurethane/Ag composite foams for improving wound healing. Biomacromolecules 2022, 23, 1622–1632.
- Jinno, C.; Morimoto, N.; Ito, R.; Sakamoto, M.; Ogino, S.; Taira, T.; Suzuki, S. A comparison of conventional collagen sponge and collagen-gelatin sponge in wound healing. BioMed Res. Int. 2016, 2016, 4567146.
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-H. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611.
- Silva, S.S.; Caridade, S.; Mano, J.; Reis, R. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr. Polym. 2013, 98, 581–588.
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365.
- Miao, J.; Pangule, R.C.; Paskaleva, E.E.; Hwang, E.E.; Kane, R.S.; Linhardt, R.J.; Dordick, J.S. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 2011, 32, 9557–9567.
- Moydeen, A.M.; Padusha, M.S.A.; Thamer, B.M.; Ahamed, N.A.; Al-Enizi, A.M.; El-Hamshary, H.; El-Newehy, M.H. Single-nozzle core-shell electrospun nanofibers of PVP/dextran as drug delivery system. Fibers Polym. 2019, 20, 2078–2089.
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin loaded poly (ε-caprolactone) nanofibers: Diabetic wound dressing with antioxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149.
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Patil, S.; Ramteke, P.W.; Laux, P.; Luch, A.; Singh, A.V. In vivo biocompatibility of electrospun biodegradable dual carrier (antibiotic+ growth factor) in a mouse model—Implications for rapid wound healing. Pharmaceutics 2019, 11, 180.
- Palo, M.; Rönkönharju, S.; Tiirik, K.; Viidik, L.; Sandler, N.; Kogermann, K. Bi-layered polymer carriers with surface modification by electrospinning for potential wound care applications. Pharmaceutics 2019, 11, 678.
- Mostafavi Esfahani, M.; Koupaei, N.; Hassanzadeh-Tabrizi, S.A. Synthesis and characterization of polyvinyl alcohol/dextran/Zataria wound dressing with superior antibacterial and antioxidant properties. J. Vinyl Addit. Technol. 2023, 29, 380–394.
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater. Sci. Eng. C 2019, 101, 306–322.
- Ghosal, K.; Chandra, A.; Roy, S.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning over solvent casting: Tuning of mechanical properties of membranes. Sci. Rep. 2018, 8, 5058.
- Habibi, S.; Mohammadi, T.; HMTShirazi, R.; Atyabi, F.; Kiani, M.; Asadi, A.A. A bilayer mupirocin/bupivacaine-loaded wound dressing based on chitosan/poly (vinyl alcohol) nanofibrous mat: Preparation, characterization, and controlled drug release. Int. J. Biol. Macromol. 2023, 240, 124399.
- Ellison, C.J.; Phatak, A.; Giles, D.W.; Macosko, C.W.; Bates, F.S. Melt blown nanofibers: Fiber diameter distributions and onset of fiber breakup. Polymer 2007, 48, 3306–3316.
- Hiremath, N.; Bhat, G. Melt blown polymeric nanofibers for medical applications-an overview. Nanosci. Technol. 2015, 2, 1–9.
- Gao, Y.; Zhang, J.; Su, Y.; Wang, H.; Wang, X.-X.; Huang, L.-P.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Recent progress and challenges in solution blow spinning. Mater. Horiz. 2021, 8, 426–446.
- Yu, B.; Han, J.; Sun, H.; Zhu, F.; Zhang, Q.; Kong, J. The Preparation and property of poly (lactic acid)/tourmaline blends and melt-blown nonwoven. Polym. Compos. 2015, 36, 264–271.
- Wang, R.; Zhang, H.; Cao, Y.; Zhai, Q.; Hu, J.; Zhen, Q.; Qian, X. Preparation of PLA/PEG@ SDS microfibers-based nonwovens via melt-blown process parameters: Wound dressings with enhanced water wetting performance. J. Appl. Polym. Sci. 2023, 140, e54234.
- Hajinasrollah, K.; Habibi, S.; Nazockdast, H. Fabrication of gelatin–chitosan–gum tragacanth with thermal annealing cross-linking strategy. J. Eng. Fibers Fabr. 2019, 14, 18033.
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144.
- de Oliveira, R.S.; Fantaus, S.S.; Guillot, A.J.; Melero, A.; Beck, R.C.R. 3D-printed products for topical skin applications: From personalized dressings to drug delivery. Pharmaceutics 2021, 13, 1946.
- Maver, T.; Smrke, D.; Kurečič, M.; Gradišnik, L.; Maver, U.; Kleinschek, K.S. Combining 3D printing and electrospinning for preparation of pain-relieving wound-dressing materials. J. Sol-Gel Sci. Technol. 2018, 88, 33–48.
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical applications of 3D printing. Addit. Manuf. 2020, 34, 101209.
- Rajesh, N.U.; Coates, I.; Driskill, M.M.; Dulay, M.T.; Hsiao, K.; Ilyin, D.; Jacobson, G.B.; Kwak, J.W.; Lawrence, M.; Perry, J. 3D-Printed Microarray Patches for Transdermal Applications. JACS Au 2022, 2, 2426–2445.
- Yeong, W.Y.; Chua, C.K.; Leong, K.F.; Chandrasekaran, M.; Lee, M.W. Indirect fabrication of collagen scaffold based on inkjet printing technique. Rapid Prototyp. J. 2006, 12, 229–237.
- Awasthi, A.; Gulati, M.; Kumar, B.; Kaur, J.; Vishwas, S.; Khursheed, R.; Porwal, O.; Alam, A.; Kr, A.; Corrie, L. Recent progress in development of dressings used for diabetic wounds with special emphasis on scaffolds. BioMed Res. Int. 2022, 2022, 1659338.
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D printing–A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42.
- Palmara, G.; Frascella, F.; Roppolo, I.; Chiappone, A.; Chiadò, A. Functional 3D printing: Approaches and bioapplications. Biosens. Bioelectron. 2021, 175, 112849.
- Penumakala, P.K.; Santo, J.; Thomas, A. A critical review on the fused deposition modeling of thermoplastic polymer composites. Compos. Part B Eng. 2020, 201, 108336.
- Sen, K.; Mehta, T.; Sansare, S.; Sharifi, L.; Ma, A.W.; Chaudhuri, B. Pharmaceutical applications of powder-based binder jet 3D printing process—A review. Adv. Drug Deliv. Rev. 2021, 177, 113943.
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424.
- Jadhav, A.; Jadhav, V.S. A review on 3D printing: An additive manufacturing technology. Mater. Today Proc. 2022, 62, 2094–2099.
- Tyagi, S.; Yadav, A.; Deshmukh, S. Review on mechanical characterization of 3D printed parts created using material jetting process. Mater. Today Proc. 2022, 51, 1012–1016.
- Rahman, M.A.; Islam, M.S.; Haque, P.; Khan, M.N.; Takafuji, M.; Begum, M.; Chowdhury, G.W.; Khan, M.; Rahman, M.M. Calcium ion mediated rapid wound healing by nano-ZnO doped calcium phosphate-chitosan-alginate biocomposites. Materialia 2020, 13, 100839.
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3D printing. Polymers 2021, 13, 598.
- Svetlizky, D.; Das, M.; Zheng, B.; Vyatskikh, A.L.; Bose, S.; Bandyopadhyay, A.; Schoenung, J.M.; Lavernia, E.J.; Eliaz, N. Directed energy deposition (DED) additive manufacturing: Physical characteristics, defects, challenges and applications. Mater. Today 2021, 49, 271–295.
- Cubo-Mateo, N.; Gelinsky, M. Wound and skin healing in space: The 3D bioprinting perspective. Front. Bioeng. Biotechnol. 2021, 9, 720217.
- Ramesh, S.; Harrysson, O.L.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting 2021, 21, e00116.
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42.
- Karvinen, J.; Kellomäki, M. Design aspects and characterization of hydrogel-based bioinks for extrusion-based bioprinting. Bioprinting 2023, 32, e00274.
- Temirel, M.; Hawxhurst, C.; Tasoglu, S. Shape fidelity of 3D-bioprinted biodegradable patches. Micromachines 2021, 12, 195.
- Masri, S.; Fauzi, M.B. Current insight of printability quality improvement strategies in natural-based bioinks for skin regeneration and wound healing. Polymers 2021, 13, 1011.
- Hao, L.; Zhao, S.; Hao, S.; He, Y.; Feng, M.; Zhou, K.; He, Y.; Yang, J.; Mao, H.; Gu, Z. Functionalized gelatin-alginate based bioink with enhanced manufacturability and biomimicry for accelerating wound healing. Int. J. Biol. Macromol. 2023, 240, 124364.
- Liao, W.; Duan, X.; Xie, F.; Zheng, D.; Yang, P.; Wang, X.; Hu, Z. 3D-bioprinted double-crosslinked angiogenic alginate/chondroitin sulfate patch for diabetic wound healing. Int. J. Biol. Macromol. 2023, 236, 123952.
