Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Mohammad Noori and Version 2 by Dean Liu.
Piezoelectric energy harvesters have recently been used in a variety of applications. They are also used in analytical chemistry for the detection and determination of various substances.
- energy harvesting
- piezoelectric materials
- analytical chemistry
1. Sorption Detector
King [1] constructed a selective and sensitive detector for gas chromatography using coated piezoelectric crystals. It is simple to measure the vibrating crystal’s frequency within 1.0 Hz., and momentous changes can be easily detected. King has shown that various substrates used in gas chromatography columns are coated with crystals and are in contact with the chromatographic components of the stream. The mass of the vapors absorbed by the coating influenced the frequency of the crystal. Also, the carrier gas has no effect on the detection limit. As a result of the combination between absorption and adsorption, King termed this device a Piezoelectric Sorption Detector. Because of its high sensitivity and selectivity, it is applicable to be used in analytical chemistry.
2. Piezoelectric Crystal Detector for Water
A wide range of materials can be used to make water vapor detectors [2]. Since 1964, hygroscopic crystal has been used as a water detector. With its high selectivity and long life, this device can detect ppm water in 30 s. Gjessing et al. have created a radio sonic element having a film of SiOx deposited over a piezoelectric crystal. Between 15 and 95 percent relative humidity, there was no hysteresis. Several studies have shown that piezoelectric crystal detectors can also be used to determine the amount of water in the Martian atmosphere. The atmosphere on the surface of Mars is different compared to that on Earth. Mariner flyby showed that the Martian atmosphere consists of 80% of carbon dioxide, with a total pressure of 6–8 mbar. There is evidence that water vapor pressure in the atmosphere of Mars ranges from 50 mm to 0.9 mm. Under these adverse conditions, King’s piezoelectric sorption hygrometers have been used to measure water vapor concentrations. Water was measured under various experimental conditions (temperature, pressure, etc.) [3][4][5]. Water measurement is an indicator that describes the level of hydration and water binding in a certain substance. Therefore, measures of water activity have great potential for technological use. In 2021 Agafonov et al. provided a technique for measuring water activity using a piezoelectric quartz crystal sensor covered with a porous layer of alumina. By injecting the air with the appropriate humidity in the vessel containing the resonator, it was possible to evaluate how the piezoelectric crystal’s frequency varies with respect to the relative humidity. The observed dependence was changed into the water sorption isotherm on the alumina. The water adsorption onto the pores wall of anodic oxide is enhanced by the presence of sulfate ions, which are the hydrated ions [6]. In order to address the global issue of sustainable access to water, Okosun et al., in 2021, developed an amino acid-based detector that is capable of detecting pipe leaks. The polycrystalline system uses the correlation between the piezoelectric voltage and the leak-induced vibration to detect pipeline degradation. The voltage constant and the piezoelectric strain for this device are 60 mV m/N and 0.9 pC/N, respectively. Compared to the PVDF patches, the crystal sensor of glycine has substantially higher sensitivity [7].
3. Detector for Gas Chromatography
With gas chromatography, the more sensitive flame ionization detector or thermal conductivity detector is used the most. Mass spectrometry and electron capture have been used to create more sophisticated detection methods. King and Karasek et al. [8][9] established a low-cost system of gas chromatography that is based on a piezoelectric crystal detector. The crystals were covered with the liquid that is used in the gas chromatography column. The compounds which are separated are detected by passing them onto the surface of a coated piezoelectric crystal, where the compound disperse within the coating of the crystal, resulting in the change in the piezoelectric crystal’s resonance. The frequency shift is used as a response to the detector and is converted to a voltage. Piezoelectric detectors can be used at room temperature or above if the carrier gas is air, nitrogen, or helium. Chromatographs can be used to apply a variety of compounds with boiling temperatures up to 200 °C. Equation (1) below shows the reaction between the coated piezoelectric crystal and the elution of the compound from the column of gas chromatograph.
(1)
Here, A represents the area of the response curve, W represents the total eluent weight, γ represents the eluent activity coefficient, P° represents eluent vapor pressure at the operating temperature, F represents the flow rate of the carrier gas, and C represents a constant which is a characteristic of the piezoelectric crystal, detector temperature, and the liquid coating over the crystal. Equation (1) describes several solvent properties of the piezoelectric crystal detectors when they are used in conjunction with the gas chromatographs. When utilizing piezoelectric crystals, the detector’s temperature is crucial. When utilized above ambient temperature, the same properties, such as linearity and sensitivity, can be seen. There are variations in the separation between the compounds observed at high temperatures because of the respective partition coefficients of the gas components. With the rising temperature, a compound’s absolute detector response declines. The result is not as dramatic as predicted. For optimal detector conditions, the detector and column temperature must be maintained as low as possible while still being higher enough for the elution of the compound of interest in a sufficient amount of time [10]. Since the vapor pressure of all chromatographic separation solvents is fixed, the lifetime of the coated crystal is determined by the solvent used and the vapor pressure of the carrier from the column. Crystals coated with polymers and adsorbents have a longer service life. If the crystal detector is damaged in an accident, it can be repaired by cleaning it with a solvent or coating it with a new substance. A detector’s lifetime is independent of the performance of the instrument. Janghorbani et al. [11] described a coated piezoelectric crystal’s response characteristic as a distribution detector of dissolved vapors in the gas stream. The partition detector theory’s authors assume that the detector is attached to the gas chromatograph column’s outlet. The response of the peak area is associated with the gas mixture of the sample. The equations developed by them to describe the action of the crystal distribution detector in the gas and liquid chromatography under equilibrium conditions are shown below (Equation (2)),
where Ay is the area below the peak caused by the y component, m represents the constant giving the change in crystal frequency caused by a mass increase at the surface, and K(y,x) is the partition coefficient of gas y in the liquid x, which describes the following ratio shown by Equation (3):
W(y,x) shows the equilibrium mass of the gas y in one unit of the crystal-coated material x, whereas Wy is the mass of the gas y in one unit of the gas phase. Vx is the volume of liquid coating x that is present on the crystal. WT indicates the mass of the gas contained in the volume of a detector when in equilibrium with a liquid coating, and F indicates the gas phase flow rate. When squalene-coated crystals were used as a detector, an admirable linear relationship between Ay and an inserted volume of pentane, hexane, and octane was noted. Table 1 shows various coatings used with piezoelectric crystal detectors in gas chromatography. To enable in-line detection in gas chromatography, Hu et al. [12] developed a revolutionary microfluidic film bulk acoustic wave resonator gas sensor (mFBAR) in 2021. The detecting element FBAR within this detector is contained within a microfluidic flow channel and operates via a desorption or adsorption mechanism. Comparative tests and computer simulations showed that adding an additional mFBAR to the capillary line (flow channel) did not significantly alter the flow or affect separation. In this system (gas chromatography-mFBAR-flame ionization detector (FID)), they showed that the simultaneous measurement of concentration profile within the mobile phase by the flame ionization detector (FID) and the direct measurement of concentration profile over the surface of the solid by mFBAR was possible. The rate of mass transfer could be easily determined by the difference between the maximum peak positions of the solid phase and the mobile phase. Figure 1b displays a 1-dimensional separation of the chromatogram for 11 gases utilizing an in-line mFBAR detector.
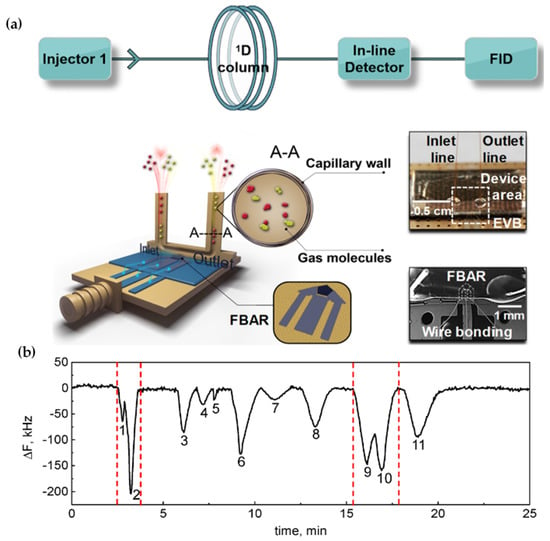
Yen et al. [13], in 2021, created an electronic nose model that is based on the surface acoustic wave to analyze the freshness and quality of the kiwifruit, Figure 2. Piezoelectric properties were displayed by LiNbO3. Due to the absorption of the volatile organic compounds, the change in frequency varied based on the characteristics of different polymers. Researchers examined the VOCs in the kiwi aroma using a thermal desorption (TD)-gas chromatograph (GC)-mass spectrometer (MS) (TD-GC-MS) system. The kiwifruit started to ripen as the esters’ concentrations rose, which was followed by an increase in the concentration and the type of VOCs. As a result, ester aroma was primarily determined using fluoropolymer and polystyrene, which acted as the sensing materials. While kiwifruits were ripening, polyvinyl butyral, poly-N-vinylpyrrolidone and polyvinyl alcohol were used to extract the acids and alcohols. The frequency shift of 2510 Hz occurred from the unripe stage to the ripe stage, and the frequency shift of 4572 Hz occurred from the ripe stage to the over-ripe stage, which is best for determining the freshness of the kiwifruit and was specifically revealed by the surface acoustic wave chip that is coated with thin polyvinyl alcohol film. This is a promising technique for identification analyses and developing food quality.
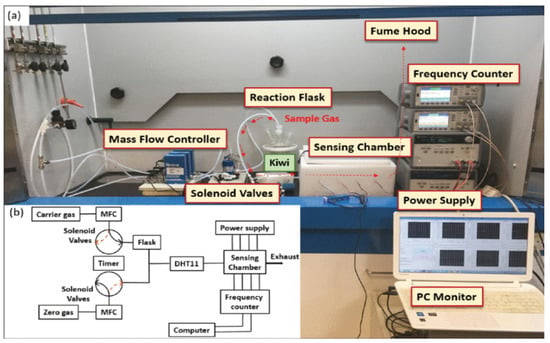
Figure 2. TD-GC-MS system, (a) experimental setup (b) schematic diagram, created by Yen et al. [13].
4. Detector for Liquid Chromatography
King and Schulz [14] constructed a worldwide mass detector for liquid chromatography. The effluent of the liquid chromatograph was sprinkled over the surface of the crystal. After the solvent has evaporated, the change in crystal frequency is used to calculate the mass of the remaining solute. Solute deposition and the sampling of liquid flow can be done quickly. Sensitivity was comparable to conventional liquid-liquid chromatography detectors, and nebulization, drying and measurement all took place in just 10 s. A gel transmission chromatograph differential refractometer directed the effluent to a crystal detector. A polystyrene blend was evaluated using chromatography, and the system was tested with butyl rubber. The mass versus retained volume distribution curves was compared with the results that were obtained with the differential refractometer detector. Refractometer detectors have been proven to be inferior to piezoelectric crystal detectors. The benefits listed by Schulz and King include that the method is non-destructive, highly sensitive, has a mass and universal detector, a wide dynamic measuring range, independent of changes in the chromatograph’s pressure temperature or flow rate, any solvent or solute mixture can be useable, volatile impurities do not inhibit it, and it is compatible with the digital response with the digital processing equipment. For effluent detection, Bastiaans and Konash [15] placed a piezoelectric crystal detector immediately in the liquid phase. A large energy loss at the interface of the liquid crystal makes it further difficult to obtain liquid phase adsorption measurements, making it more difficult to sustain crystal vibrations. The piezoelectric crystal’s frequency is determined by the liquid phase density over the crystal’s surface. Therefore, the change in density induced by the solvent gradient and the solute causes the crystal’s resonant frequency to drift. Reference crystals and a coated sample crystal were used to compensate for changes in liquid density. Only one side of each crystal encounters the liquid phase to achieve stable oscillations. The density gradient, flow rate, and temperature effects are removed by using a reference crystal. To regulate the surface adsorption capabilities of the sample crystals in the presence of effluent, long-chain hydrocarbons were used. These coatings could only detect smaller non-polar molecules; however, advanced surface modification techniques offer more sensitive and rapid detection. Piezoelectric crystals give very good results when they are used in photoacoustic detectors for liquid chromatography. According to the photoacoustic detection principle, when a solute is dissolved in the solvent and irradiated with a suitable light source, the solution expands due to light adsorption. Expansion to a piezoelectric crystal changes crystal resonant frequency. When a laser is utilized to illuminate the sample, photoacoustic detection of the compounds in the static solutions becomes an extremely sensitive approach. Therefore, an analysis of this type of detection system’s application in liquid chromatography was analyzed. By combining liquid chromatography with the quartz crystal microbalance, Kartanas et al. [16] describe a technique for label-free protein analysis. This method uses size omission chromatography to initially separate a mixture of proteins in the physiological buffer solution, allowing for the selection of particular protein fractions, desalination, and subsequent spray-drying over the quartz crystal microbalance for mass analysis. Protein detection and sample fractionation are accomplished simultaneously by creating a continuous interface between the spray device and chromatography column using the flow splitter with precision as low as 100 μg/mL. With this method of quantitative mixture analysis, it could be possible to identify different protein species within physiological conditions. The most widely employed bulk analytical techniques have limitations regarding their capability to separate particular protein species hence needing more time to take steps. This work demonstrates a method for addressing these challenges by executing label-free protein detection by combining liquid chromatography with the gravimetric quartz crystal microbalance detection using the micro-fluidic spray nozzle and resulting in the protein detection limit at the microgram level.
5. Trace Metal Analysis
Mieure and Jones [17] created an electrogravimetry assay that uses piezoelectric crystals to determine trace metals in solution. The electrochemical cell’s cathode was made of AT-cut quartz. After passing the known amount of current through the cell, the crystals were removed, washed, and dried. The concentration was calculated by utilizing the frequency shift caused by metal deposition. Cadmium solutions with concentrations ranging from 5.0 × 10−4 M to 5.0 × 10−8 M were investigated. Cadmium accurateness of this technique ranged from 0.42% at a large concentration to 8.7% at a small concentration. Nomura and Mimatsu [18] measured iodide in solution using another electro-gravimetric analysis using silver-covered piezoelectric crystals on a platinum-coated gold electrode. Iodide was electrodeposited at −0.05 Volts in a 10−3 M potassium chloride (KCl) sample solution. The pH of the sample solution was adjusted to 9.8 with 10−3 M sodium tetraborate (IH) caustic soda solution. This technique involves first running the reagent blank through the detection cell until it reaches a specific frequency. Iodide was present in the sample solution, which was then passed for 1 min (10−6 M to 10−5 M) or 10 min (10−7 M to 10−6 M). After each assay, −0.4 V electrolysis can be used to remove the iodide from the crystal. Lead was extracted from the solution using platinum-coated piezoelectric crystals. In another report, Nomura and Maruyama [19] examined the stability of metal ions in solutions and the method for detecting iron (III) as a phosphate. For concentrations up to 2 mM, it has been demonstrated using standard piezoelectric crystals that the measured frequency change of metals in solution is proportional to the specific conductance, with variations owing to solution density and viscosity. The solution short-circuiting the crystal caused significant frequency changes at concentrations above 20 mM. Under these conditions metal ions i.e., Ni2+, Mn2+, Zn2+, Co2+, Cd2+, Pb2+, Ag2+, and Cu2+ was deposited on the electrodes. Changes in aluminum and iron frequencies have been attributed to salt adsorption. Another quartz plate was placed on the other side of the quartz plate, with just “four thin hairs deposited on each of the sides of the ‘square’ quartz plate”, separating the two. The plate was firmly bonded with epoxy resin. To avoid electrolysis, resin is also applied to the crystal holder’s lead wire. It was discovered that the modified crystal responded linearly to lead (III) in the range of 1 × 10−5 M to 1 × 10−4 M. Interference experiments concerning an iron (III) solution at 5 × 10−5 M have shown that the 10-fold molarities can be harmful if deviations larger than 15% are attained. This occurs with aluminum, sulfide and thiosulphate, bismuth, and lead [3][4][5]. The contamination brought on by the existence of trace metals that could transmit through the in-filtrate water and soil is one of the main problems with mining operations and has a direct impact on the sustainability index for the environment. There are numerous methodologies that could be utilized to detect trace metals in the water and soil, as depicted in Figure 3, but the majority of them have limitations that make it impossible to apply them in real-world settings. There have been numerous attempts to develop portable sensor devices for regulating environmental trace metal concentrations. The transducer and the sensing elements are the two primary components of a traditional sensor for the precise detection of an analyte. This is because adding new structures to sensors, like nanostructures, can significantly increase their effectiveness in terms of selectivity, sensitivity, portability, and multiplexed detection [20]. Due to their availability, chemical stability, and high-temperature impedance, quartz crystals have become the most often used type of natural and synthetic materials that demonstrate the piezoelectric effect [21]. The self-generating, flexibility, high frequency, huge dielectric constant, and ease of use are some of the benefits of piezoelectric transducers.
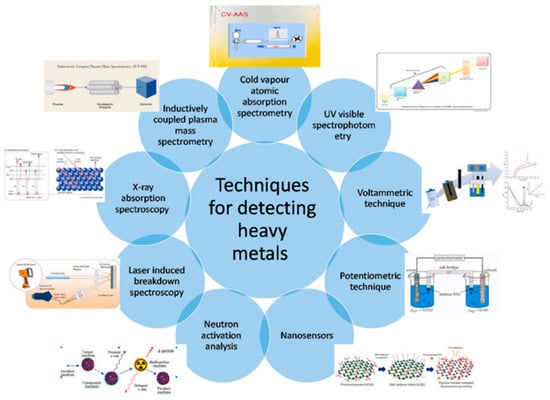
Figure3. Numerous methodologies utilized for the detection of trace metals reproduced with permission from [20], Elsevier, 2021.
The quartz crystal microbalance could be utilized under a variety of conditions, including liquid conditions, gas sensors, and in vacuums. It is helpful for monitoring the record of the rate of vacuum-based thin-film deposition systems. A flow-type chemical sensor was investigated by Sartore et al. [22] in 2011 for the purpose of detecting trace metals in the aqueous solutions. The 9 MHz AT-cut quartz crystal resonator used in the sensor’s construction enables the surface chelation of metal ions. The use of new methods has made it possible to isolate gold electrodes with surface modifications that are highly capable of tracing metal ions and complexing them. By complexing with the functional groups included in polymers, these polymers grafted quartz crystal microbalance sensors may effectively adsorb trace metals from solution, such as lead, chrome, cadmium, and copper, in a range between 0.01 ppm–1000 ppm of concentration.
Similarly, Huseynli et al. [23], in 2018, investigated a novel method for the quartz crystal microbalance nano-sensors for the detection of Hg (II) ions in wastewater. In this method, the N-methacryloyl-(l)-cysteine (MAC) and the Hg2+ ions were transformed in the pre-complex, which was subsequently changed on the nano-sensor chips to produce pHEMAC polymers and pHEMAC-Hg (II) ions. According to the estimates, the detection limit is about 0.21 × 10−9 M. The developed Hg (II) ions imprinted nano-sensors exhibit great sensitivity and selectivity for the detection of Hg (II) ions from wastewater. This approach outperforms others in terms of speed, sensitivity, and affordability.
6. Detection of Viruses
Various viruses such as human papilloma, dengue virus, vaccinia, influenza A virus, Ebola virus, hepatitis B, and human immunodeficiency virus are detected by piezoelectric sensors. An alternating current causes a piezoelectric material to mechanically oscillate, creating an oscillating electrical field. The frequency regulated by alternating current voltage drops when the mass rises as a result of the molecular interactions. Piezoelectric sensors of the mass response type are frequently employed for the detection of viruses. Figure 4 provides a schematic representation of the piezoelectric biosensor’s working. On the piezoelectric material’s upper surface of the electrode, antibodies are attached. The piezoelectric material resonates as a result of the top and bottom electrodes. The majority of materials utilized for sensor materials are anisotropic, including PVDF, BaTiO3, PbTiO3, ZnO, AIN, and SiO2 [24].
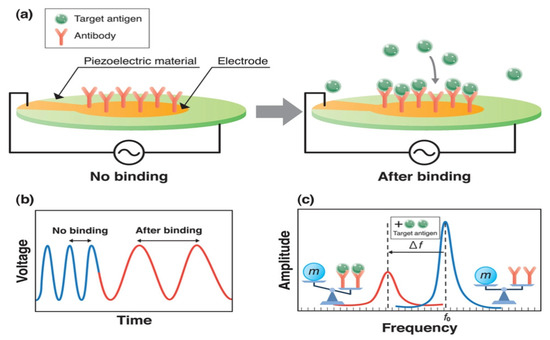
Figure 4. Piezoelectric material used for virus detection; (a) piezo-electric biosensor operating principle; graph of (b) voltage to time and (c) amplitude to frequency, during detection [24].
Human papillomavirus is a potential cause of cervical cancer, the third-most frequent malignancy in women. Fu et al. [25] created a piezoelectric gene sensor for the detection of the papillomavirus using an AT-cut quartz crystal of 10 MHz. Airborne detection and the rapid detection of the vaccinia virus were achieved using quartz crystal microbalance technology. By integrating the quartz crystal microbalance detection methods and polymerase chain reaction (PCR) amplification, Kleo et al. [26] produced a unique system for the detection of the vaccinia virus. Dengue fever is a widespread viral disease that is spread by mosquitoes and causes thousands of fatalities each year. It is a serious health issue in urban and semi-urban areas. A piezoelectric immuno-chip was created by Wu et al. [27] for the detection of the dengue virus. They utilized a 10 MHz quartz crystal microbalance to detect dengue E protein and NS-1 protein using an 8 mm AT-cut quartz wafer sandwiched between the Au electrodes. Since the discovery of the Ebola virus in 1976, thousands of individuals have died from the illness. The virus is transferred among humans via direct contact with the secretions, blood, other body fluids, or organs of the infected persons, as well as with the surfaces and materials that have been contaminated by these fluids. This virus is spread in humans from wild animals. For the quick detection of the Ebola virus, Baca et al. [28] suggested a label-free sensing method that is based on the surface acoustic wave sensor. The sensor chips were created by utilizing wafers of LiTaO3. The influenza virus is the most harmful and prevalent infection, which has a high level of infectivity and mutagenicity. Type A virus is transmitted both from human to human and animal to human. For the purpose of detecting the influenza-A virus, Jiang et al. [29] devised and created a surface acoustic wave sensor with piezoelectric LiNbO3 wafers coated with SiO2. Another of the most frequently encountered diseases around the world is infection with hepatitis B. Despite the fact that billions of individuals have the hepatitis virus, effective treatments and medications have still not been developed to treat chronic hepatitis-B infections. Using the micro-fabrication technology, Xu et al. [30] created a piezo diaphragm-based immuno-assay chip to identify the anti-hepatitis B virus. The results showed a detection limit of 0.1 ng/mL.
7. COVID-19 Detection
People’s lives have changed all around the world as a result of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) outbreak, which has had a profound effect on economies and communities.
By 2030, it is predicted that the IoTs and AI will have a significant economic influence and become progressively more in demand in the upcoming post-corona society. For instance, AI-enabled IoT-connected biosensors may proliferate. Currently, there is a need for the creation of reliable and effective piezoelectric biosensors that can detect SARS-CoV-2. In fact, it has been reported that SARS-CoV can be detected using a piezoelectric immuno-sensor [31] and applying the recently suggested surface chemistry to the surface of quartz crystal of the quartz crystal microbalance will enable the rapid detection of the SARS-CoV-2 [32].
The spike protein has hydrophobic and positively charged residues of amino acids at its protein binding sites. As a result, it is anticipated that a surface that is hydrophobic and negatively charged will adsorb or attach to the spike protein because of the strong electrostatic and hydrophobic interactions. The best-engineered surface used for this purpose appears to be mixed self-assembled monolayers of COOH and CH3 groups. For the real-time detection of the SARS-COV-2 with a sensitivity up to the ng range, the described surface chemistry could be applied to the quartz crystal surface of the quartz crystal microbalance. A piezoelectric microcantilever biosensor was developed for the immediate detection of COVID-19 without the need for any pre-treatment. The associated antibody is coated on the biosensor, which serves as a transducer. A piezo-electric microcantilever bio-sensor function based on the interactions between SARS-CoV-2 antigen and antibody. Through their spike proteins, the SARS-CoV-2 antigens adhered/attached to the microcantilever top surface. Different piezoelectric materials were assessed in order to create a biosensor with the optimum parameters. Consequently, it was determined that a PVDF biosensor provided the optimal result. As a result, the rapid detection of COVID-19 in clinical samples with different viral loads is made possible by the extremely sensitive microcantilever biosensor [33]. Piezoelectrics are attractive candidates for self-powering biosensors for the detection of viruses and communication by harvesting energy from environmental sources. Wearable biosensors should be integrated with the energy harvesters to provide a self-power source. By automatically gathering and transmission of data, such multi-function self-powered devices may be able to control health, making it possible for people to live without the fear of acquiring numerous viral infections. A representation of this type of future society is seen in Figure 5 [24]. Wearable actuators might be capable of guiding individuals away from dangerous situations. The energy required could be generated by the breeze. AI may be able to anticipate the severity and the rate of disease spread.
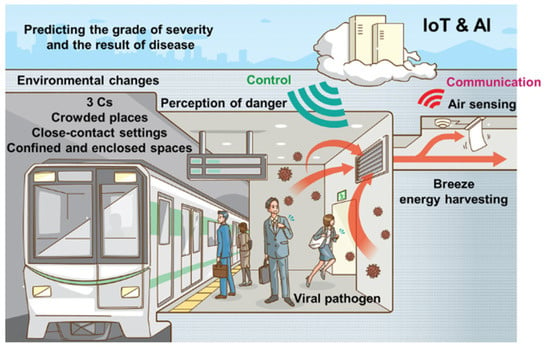
Figure 5. Future society [24].
8. Detector for Air Pollutant Detection and Determination
For over 60 years, analyzing SO2 in the air has been a great concern. Oil refineries, paper and pulp mills, and the effluents released from a variety of other industries are all major sources of SO2 emissions into the environment. Another ground-level pollution is the combustion of high-sulfur fuels in automobiles. As a result, there is a growing demand for new, effective, simple, and low-cost methods for measuring and controlling SO2 pollution. Several scientific studies [33][34][35][36][37][38][39][40][41] have described the use of coated piezoelectric crystals as sensitive SO2 detectors. For SO2, many coating materials have been studied. A novel detector was designed using triethanolamine and quadrol as the coating materials, which may detect trace quantities of SO2 [42]. The primary characteristic of this system is the separation of the column effluent into two equal streams that fall on the opposite faces of a coated crystal simultaneously and directly. This design is expected to enhance sensitivity since the amount of the sample gas reacting with the coating at any specific time is greatly increased. Several experiments examined the effect of changing the temperature. With rising the temperature, the crystals’ frequency also increased, according to Guilbault et al. [34], especially from 100 °C to 200 °C. The influence of temperature increases just slightly from 25 °C to 40 °C at 40 Hz. According to these experiments, the temperature needs to be consistent during the reading, but an increase in temperature of 10 degrees Celsius is not critical. Cheney et al. [35] employed triethanolamine as a coating substance. They found that an uncoated 9 MHz crystal’s temperature dependence changed by 71 Hz when the temperature was raised from 10 °C to 35 °C. Investigations were also conducted on the SO2 desorption and adsorption of the coated material at various temperatures. Several diverse ways of applying the substrate to the piezoelectric crystal were examined. Spraying, dropping, and dipping techniques were among them. According to Hartigan [43], the most important factor in coating the crystal is the capability to reproduce the coating procedure, not the amount of coating. For SO2 detection, Cheney et al. [33] utilized cotton swabs to cover a crystal with ethylenedinitrotetraethanol. A center-covered 9 MHz crystal (340 Hz) was shown to be more sensitive to SO2 as compared to the fully coated crystal (260 Hz). The scientists also found that the frequency variation caused by varying coating is predictable and constant for a center-coated crystal but not for a fully-coated crystal.
Coated piezoelectric crystal detectors were used to detect NH3 in the ppb range. A great sensitivity towards ammonia was obtained when the coatings Ucon-LB-3OOX and Ucon 75-H-90,000 were applied [44]. The Ucon coatings reacted with the nitrogen dioxide to create new compounds upon that crystal, showing a high sensitivity towards both nitrogen dioxide and ammonia. These chemicals’ infrared spectra showed the creation of new bands and modifications to certain existing bands, indicating the synthesis of new molecules. Atmospheric moisture and excessive amounts of organic chemicals that disintegrate the coatings caused several problems. New coating materials, i.e., coating of ascorbic acid, coating of capsicum annuum pods, and coating of ascorbic acid with silver nitrate, have been used to detect ammonia in the atmosphere precisely [45].
The impact of pesticides on the environment has become a major issue in recent years. Because they are strong cholinesterase inhibitors, pesticides are hazardous to both people and animals. The reactions that the organophosphorus pesticides undergo are all structurally connected to one another. Di-isopropylmethyl phosphonate (DIMP) was selected as a model compound in research by Guilbault and Scheide because almost all organophosphorus pesticides comprise either phosphoryl or thiophosphoryl groups, and the thiophosphoryl pesticides easily undergo oxidation reactions to produce phosphoryl containing compounds [46]. Various inorganic salts, i.e., CuCl2, CdCl2, NiCl2, and FeCl3, were used to coat the crystal, which had an impact on the detection of DIMP in the ppm range. It was determined that other organophosphorus compounds with a similar structure could not be detected using the FeCl3-DIMP complex, which has been utilized as the substrate to measure low quantities of DIMP. In order to specifically determine paraoxon levels, a detector was developed utilizing a piezoelectric crystal and a FeCl3- paraoxon complex as the substrate. Various inorganic salts, i.e., HgBr2, CuC12, HgCl2, MnC12, ZnCl2, and MoCl5, had a strong chemical interaction with DIMP [47]. Guilbault noted that salts of the majority of transition metals should perform well as piezoelectric crystal detector coatings.
Hydrogen sulfide is a hazardous gas that raises safety concerns for many industries. This is particularly true as dangerous levels of hydrogen sulfide can go unnoticed by workers and rise suddenly. Techniques have been developed to detect hydrogen sulfide in the atmosphere [48]. The adsorption of hydrogen sulfide (H2S) on a crystal surface that has been covered with an acetone extract of different soot particles that are produced when specific organochlorine chemicals are burned is the basis of this technique. The best substrate was chlorobenzoic acid extract of soot, and this technique is highly effective in the concentration range between 1–60 ppm. Lead acetate, copper and silver metal, and anthraquinone disulfonic acid are additional coating materials proposed by King to detect hydrogen sulfide.
9. Solution Measurement
Nickel dimethylglyoxime has been utilized as a coating by Webber and Guilbault [44] to detect ammonia in solution. By Utilizing the hydrophobic membranes between both the sample solution as well as the crystal, the effects of moisture were reduced. Alternately, the crystal was moved to the sampling chamber after being allowed to establish equilibrium over a sample of the distilled water. A signal of -135 Hz was produced by 0.15 M of ammonia in water. Up to a value of 0.45 M ammonia, the calibration curve was linear. Sulfur dioxide was determined in the ppb range under identical circumstances utilizing quadrol as the coating.
Piezoelectric crystals have been utilized by Nomura and colleagues [49][50] to detect cyanide in solution. AT crystals cut to 9 MHz gold electrodes with silver plating were utilized. An original investigation either used dihydrogen phosphate-borate or a borate-hydroxide buffer to maintain a consistent volume of a sample or a standard solution at pH 9.6. In a water bath, the solution was maintained at a consistent temperature of 25 °C. For the analysis, the crystal, whose frequency was already determined, was submerged inside this solution for about 15 min while being agitated at 430 rpm. After being removed, the crystal was cleaned with acetone and water before being immersed in a steady stream of 30 °C air. The frequency was determined after 1 minute. According to reports, cyanide measurement has a linear range from 10−7 M to 10−5 M. Ethylenediaminetetraacetic acid (EDTA) was used to remove the cation interferences that formed complexes with cyanide.
A preliminary investigation into the utilization of the piezoelectric quartz crystals to evaluate the rate of microbial or fungal growth on their surface was published in Down [51]. The study was unable to quantify any significant alterations brought on by the expansion of these biological systems. It was believed that cells grew very slowly and that the cell membrane was broken by high-frequency vibrations.
Nomura and Tsuge [52] used an oscillator equipped with transistors to develop a technique for the determination of the silver concentration in the solution. It was found that this form of oscillator had a much lower frequency drift than an integrated circuit oscillator, which has previously been reported [53]. Silver concentration in solution is measured using a three-electrode deposition system that includes a platinum-plated gold electrode as that of the cathode, a coiled platinum wire as the anode, and a silver chloride as the reference electrode. The test solution is mixed with 1 × 10−3 M EDTA to create a stable combination with the intervening ions in the solution. Mercury (II) was deposited on the electrode even in the presence of EDTA; however, a technique was established for both silver and mercury in the solution. After 10 min of electrodeposition, the silver response was linear, ranging from 10−6 M to 3 × 10−5 M and from 2 × 10−7 M to 1 × 10−6 M after 1 h.
Nomura and colleagues [54] devised a sensitive method for the determination of iodide in the solution utilizing a silver-plated piezoelectric crystal. First, 11.2 mL (approximately 0.38 oz)/min of a blank solution containing reagent is delivered through the cell until the crystal’s frequency has stabilized (F1). The blank solution containing the reagent is added to the cell till the crystal reaches equilibrium (F2) after the sample or the standard solution has been running through it for precisely five minutes. The frequency change (ΔF), i.e., F = F1 − F2 is proportionate to the concentration of iodide. A 0.01 M of ammoniacal buffer solution having pH 9.4 containing 2 mM of sodium thiosulfate can then be passed through the cell for over 30 s at 50 Hz to remove the deposited iodide from the electrode (F). At least 30 determinations can be performed before the electrode must be replotted. This technique can detect iodide at concentrations ranging from 0.5 M to 7 M.
10. Miscellaneous Applications
Daley et al. [55] determined the mass (concentration) of an aerosol using a piezoelectric crystal sensor. Humidity, temperature, particle accumulation characteristics, mass sensitivity, and response linearity were examined as five areas of influence. No reference crystal is used to compensate for changes in airflow temperature or humidity. By reducing the inlet temperature’s rate of change, the error due to temperature was successfully reduced. The absorption and release of moisture by aerosol deposition caused the error due to humidity. The recorded linear response limitations for several aerosols and devices ranged from 0.2 µg/mm2 to 6 µg/mm2. Mass sensitivity was affected by sediment size and location. At particle sizes of 2 m in diameter, mass sensing was reduced, reaching 0 m to 20 m. In the size range of 2 μm–20 μm, plastic crystal coatings improved sensor performance. Olin et al. [56] assert that the suspended particle’s mass concentration can be determined using piezoelectric quartz as a microbalance. A collector, such as an electrostatic precipitator or an impactor, deposits suspended particles on the surface of the electrode of the vibrating crystal, and the resonance frequency lowers proportionally to the additional mass of the particles. The device’s time resolution and high sensitivity were examined. An electrostatic precipitator that sampled at 1 L/min was utilized to measure the mass concentration of the particles in the air at 41 s to within 5%. Chuan [57] s described a portable direct reading device capable of monitoring particle mass concentrations in the range of about 50 µg/m3 to 5000 µg/m3. Vapors in the air, including water vapor, have no impact on the sensors. The chemisorption reactions of mono-, dimethyl-, and trimethylamine with piezoelectric crystals in vacuum systems have been studied at ambient temperature [58][59]. For the solid substrate coating over the crystals, thin films of several metal salts such as ZnCl2, FeCl3, CoCl2, ZnI2, and HgBr2 have been applied. The reactions were studied to find the optimal coating for detecting and identifying these dangerous amines with piezoelectric crystal detectors. Iron (III) chloride coating has immense potential. Behrndt etc. [60] and Oberg [61] created a technique that makes use of piezoelectric crystals to measure the deposition rate and the thickness of the film. AT-cut crystals in thick slip mode with a vibration frequency of 2.5 MHz show a frequency variation of about 1 Hz per thickness of the metal accumulated over the exposed facets. On average, crystals can hold about 20,000 g (about 44.09 lb) of metal before the deposits need to be removed or new crystals need to be used. King [62] used a solvent sorption detector to create a simple portable detector for studying the detection limits of various chemicals. Finally, quartz thermometers, quartz pressure transducers [63], and thin film thermocouples [64] are some other applications.
References
- King, W.H. Piezoelectric Sorption Detector. Anal. Chem. 1964, 36, 1735–1739.
- Lee, C.; Fung, Y.; Fung, K. A piezoelectric crystal detector for water in gases. Anal. Chim. Acta 1982, 135, 277–283.
- Sheeraz, M.A.; Butt, Z.; Khan, A.M.; Mehmood, S.; Ali, A.; Azeem, M.; Nasir, A.; Imtiaz, T. Design and Optimization of Piezoelectric Transducer (PZT-5H Stack). J. Electron. Mater. 2019, 48, 6487–6502.
- Guilbault, G.G.; Jordan, J.M.; Scheide, E. Analytical Uses of Piezoelectric Crystals: A Review. CRC Crit. Rev. Anal. Chem. 1988, 19, 1–28.
- Lu, C.; Czanderna, A.W. Applications of Piezoelectric Quartz Crystal Microbalances; Elsevier Science: Burlington, NJ, USA, 2012.
- Shahid, M.; Javed, H.M.A.; Ahmad, M.I.; Qureshi, A.A.; Khan, M.I.; Alnuwaiser, M.A.; Ahmed, A.; Khan, M.A.; Tag-ElDin, E.S.M.; Shahid, A.; et al. A Brief Assessment on Recent Developments in Efficient Electrocatalytic Nitrogen Reduction with 2D Non-Metallic Nanomaterials. Nanomaterials 2022, 12, 3413.
- Okosun, F.; Guerin, S.; Celikin, M.; Pakrashi, V. Flexible amino acid-based energy harvesting for structural health monitoring of water pipes. Cell Rep. Phys. Sci. 2021, 2, 100434.
- Karasek, F.; Tiernay, J. Analytical performance of the piezoelectric crystal detector. J. Chromatogr. A 1974, 89, 31–38.
- Karasek, F.; Guy, P.; Hill, H.; Tiernay, J. Chromatographic design and temperature-related characteristics of the piezoelectric detector. J. Chromatogr. A 1976, 124, 179–186.
- Drake, P. The Development of Quartz Crystal Microbalance Based Chemical Sensors. Ph.D. Dissertation, University of Bath, Bath, UK, 2000.
- Edmonds, T.; West, T. A quartz crystal piezoelectric device for monitoring organic gaseous pollutants. Anal. Chim. Acta 1980, 117, 147–157.
- Hu, J.; Qu, H.; Pang, W.; Duan, X. In-Line Detection with Microfluidic Bulk Acoustic Wave Resonator Gas Sensor for Gas Chromatography. Sensors 2021, 21, 6800.
- Yen, T.-Y.; Yao, D.-J. Detection of the Freshness of Kiwifruit With a TD-GC-MS and a Gas-Sensing Array Based on the Surface-Acoustic-Wave Technique. IEEE Trans. NanoBiosci. 2021, 21, 363–369.
- Konash, P.L.; Bastiaans, G.J. Piezoelectric crystals as detectors in liquid chromatography. Anal. Chem. 1980, 52, 1929–1931.
- Oda, S.; Sawada, T. Laser-induced photoacoustic detector for high-performance liquid chromatography. Anal. Chem. 1981, 53, 471–474.
- Kartanas, T.; Levin, A.; Toprakcioglu, Z.; Scheidt, T.; Hakala, T.A.; Charmet, J.; Knowles, T.P. Label-free protein analysis using liquid chromatography with gravimetric detection. Anal. Chem. 2021, 93, 2848–2853.
- Jones, J.L.; Mieure, J.P. Piezoelectric transducer for determination of metals at the micromolar level. Anal. Chem. 1969, 41, 484–490.
- Nomura, T.; Mimatsu, T. Electrolytic determination of traces of iodide in solution with a piezoelectric quartz crystal. Anal. Chim. Acta 1982, 143, 237–241.
- Nomura, T.; Maruyama, M. Effect of metal ions on a piezoelectric quartz crystal in aqueous solution and the adsorptive determination of iron(III) as phosphate. Anal. Chim. Acta 1983, 147, 365–369.
- Ekrami, E.; Pouresmaieli, M.; Shariati, P.; Mahmoudifard, M. A review on designing biosensors for the detection of trace metals. Appl. Geochem. 2021, 127, 104902.
- Eddaif, L.; Shaban, A.; Telegdi, J. Sensitive detection of heavy metals ions based on the calixarene derivatives-modified piezoelectric resonators: A review. Int. J. Environ. Anal. Chem. 2019, 99, 824–853.
- Sartore, L.; Barbaglio, M.; Borgese, L.; Bontempi, E. Polymer-grafted QCM chemical sensor and application to heavy metal ions real time detection. Sens. Actuators B Chem. 2011, 155, 538–544.
- Hüseynli, S.; Çimen, D.; Bereli, N.; Denizli, A. Molecular Imprinted Based Quartz Crystal Microbalance Nanosensors for Mercury Detection. Glob. Chall. 2018, 3, 1800071.
- Narita, F.; Wang, Z.; Kurita, H.; Li, Z.; Shi, Y.; Jia, Y.; Soutis, C. A Review of Piezoelectric and Magnetostrictive Biosensor Materials for Detection of COVID-19 and Other Viruses. Adv. Mater. 2020, 33, e2005448.
- Huang, J.; Chen, B.; Fu, W.; Huang, Q.; Wang, J.; Liu, M. Detection of Human Papilloma Virus with Piezoelectric Quartz Crystal Genesensors Superconductivity and Quantum Phase Transition View project Ras localization and its relative function View project Detection of Human Papilloma Virus with Piezoelectric Quartz Crystal Genesensors. Sens. Transducers Mag. (S&T e-Dig.) 2004, 42, 214–219. Available online: http://www.sensorsportal.com (accessed on 25 October 2022).
- Kleo, K.; Kapp, A.; Ascher, L.; Lisdat, F. Detection of vaccinia virus DNA by quartz crystal microbalance. Anal. Biochem. 2011, 418, 260–266.
- Wu, T.-Z.; Su, C.-C.; Chen, L.-K.; Yang, H.-H.; Tai, D.-F.; Peng, K.-C. Piezoelectric immunochip for the detection of dengue fever in viremia phase. Biosens. Bioelectron. 2005, 21, 689–695.
- Baca, J.T.; Severns, V.; Lovato, D.; Branch, D.W.; Larson, R.S. Rapid Detection of Ebola Virus with a Reagent-Free, Point-of-Care Biosensor. Sensors 2015, 15, 8605–8614.
- Jiang, Y.; Tan, C.Y.; Tan, S.Y.; Wong, M.S.F.; Chen, Y.F.; Zhang, L.; Yao, K.; Gan, S.K.E.; Verma, C.; Tan, Y.-J. SAW sensor for Influenza A virus detection enabled with efficient surface functionalization. Sens. Actuators B Chem. 2014, 209, 78–84.
- Xu, T.; Miao, J.; Wang, Z.; Yu, L.; Li, C.M. Micro-piezoelectric immunoassay chip for simultaneous detection of Hepatitis B virus and α-fetoprotein. Sens. Actuators B Chem. 2011, 151, 370–376.
- Zuo, B.; Li, S.; Guo, Z.; Zhang, J.; Chen, C. Piezoelectric Immunosensor for SARS-Associated Coronavirus in Sputum. Anal. Chem. 2004, 76, 3536–3540.
- Pandey, L.M. Design of engineered surfaces for prospective detection of SARS-CoV-2 using quartz crystal microbalance-based techniques. Expert Rev. Proteom. 2020, 17, 425–432.
- Cheney, J.; Norwood, T.; Homolya, J. The Detection of Sulfur Dioxide Utilizing a Piezo-Electric Crystal Coated with Ethylenedinitrilotetraethanol. Anal. Lett. 1976, 9, 361–377.
- Guilbault, G.G.; Lopez-Roman, A. Use of Sodium Tetrachloromercuriate as a Substrate for the Determination of So2 on the Piezocrystal Detector. Environ. Lett. 1971, 2, 35–45.
- Cheney, J.L.; Homolya, J.B. The development of a sulfur dioxide continuous monitor incorporating a piezo-electric sorption detector. Sci. Total Environ. 1976, 5, 69–77.
- Wade, W.H.; Slutsky, L.J. Adsorption on Quartz Single Crystals. In Vacuum Microbalance Techniques; Springer: Boston, MA, USA, 1962; pp. 115–128.
- Guilbault, G.G.; Lopez-Roman, A.; Billedeau, S. Gas-phase reactions of mono-, di-, and trimethylamine with various metal salts: The use of piezoelectric crystals in a vacuum system. Anal. Chim. Acta 1972, 58, 421–427.
- Karmarkar, K.H.; Webber, L.M.; Guilbault, G.G. Measurement of SO 2 in Air Using Coated Piezoelectric Crystal Detectors. Environ. Lett. 1975, 8, 345–352.
- Karmarkar, K.; Guilbault, G. A new design and coatings for piezoelectric crystals in measurement of trace amounts of sulfur dioxide. Anal. Chim. Acta 1974, 71, 419–424.
- Frechette, M.W.; Fasching, J.L.; Rosie, D.M. Evaluation of substrates for use on a piezoelectric detector for sulfur dioxide. Anal. Chem. 1973, 45, 1765–1766.
- Frechette, M.W.; Fasching, J.L. Simple Piezoelectric Probe for Detection and Measurement of SO2. Environ. Sci. Technol. 1973, 7, 1135–1137.
- Webber, L.; Karmarkar, K.; Guilbault, G. A coated piezoelectric crystal detector for the selective detection and determination of hydrogen sulfide in the atmosphere. Anal. Chim. Acta 1978, 97, 29–35.
- Hlavay, J.; Guilbault, G.G. Applications of the piezoelectric crystal detector in analytical chemistry. Anal. Chem. 1977, 49, 1890–1898.
- Webber, L.M.; Guilbault, G.G. Coated piezoelectric crystal detector for selective detection of ammonia in the atmosphere. Anal. Chem. 1976, 48, 2244–2247.
- Tomita, Y.; Guilbault, G.G. Coating for a piezoelectric crystal sensitive to organophosphorus pesticides. Anal. Chem. 1980, 52, 1484–1489.
- Suleiman, A.; Guilbault, G. Piezoelectric Crystal Detectors for Environmental Pollutants. Stud. Environ. Sci. 1994, 59, 273–303.
- Shackelford, W.M.; Guilbault, G.G. A piezoelectric detector for organophosphorus pesticides in the air. Anal. Chim. Acta 1974, 73, 383–389.
- Scheide, E.P.; Guilbault, G.G. Piezoelectric detectors for organophosphorus compounds and pesticides. Anal. Chem. 1972, 44, 1764–1768.
- Nomura, T.; Hattori, O. Determination of micromolar concentrations of cyanide in solution with a piezoelectric detector. Anal. Chim. Acta 1980, 115, 323–326.
- Nomura, T. Single-drop method for determination of cyanide in solution with a piezoelectric quartz crystal. Anal. Chim. Acta 1981, 124, 81–84.
- Morris, R.G.; Downes, J.J.; Sahakian, B.J.; Evenden, J.L.; Heald, A.; Robbins, T.W. Planning and spatial working memory in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 757–766.
- Nomura, T.; Okuhara, M. Frequency shifts of piezoelectric quartz crystals immersed in organic liquids. Anal. Chim. Acta 1982, 142, 281–284.
- Nomura, T.; Tsuge, K. Determination of silver in solution with a piezoelectric detector after electrodeposition. Anal. Chim. Acta 1985, 169, 257–262.
- Nomura, T.; Watanabe, M.; West, T. Behaviour of piezoelectric quartz crystals in solutions with application to the determination of iodide. Anal. Chim. Acta 1985, 175, 107–116.
- Daley, P.S.; Lundgren, D.A. The Performance of Piezoelectric Crystal Sensors Used to Determine Aerosol Mass Concentrations. Am. Ind. Hyg. Assoc. J. 1975, 36, 518–532.
- Olin, J.G.; Sem, G.J. Piezoelectric microbalance for monitoring the mass concentration of suspended particles. Atmos. Environ. (1967) 1971, 5, 653–668.
- Liu, B.Y. Fine Particles: Aerosol Generation, Measurement, Sampling and Analysis; Academic Press: New York, NY, USA, 1976.
- Karmarkar, K.; Webber, L.; Guilbault, G. Measurement of sulfur dioxide in automobile exhausts and industrial stack gases with a coated piezoelectric crystal detector. Anal. Chim. Acta 1976, 81, 265–271.
- Tomita, Y.; Ho, M.H.; Guilbault, G.G. Detection of explosives with a coated piezoelectric quartz crystal. Anal. Chem. 1979, 51, 1475–1478.
- Behrndt, K.; Love, R. Automatic control of film-deposition rate with the crystal oscillator for preparation of alloy films. Vacuum 1962, 12, 1–9.
- Oberg, P.; Lingensjo, J. Crystal Film Thickness Monitor. Rev. Sci. Instrum. 1959, 30, 1053.
- Richardson, P.D. Discussion of paper by W. H. King., Jr.: The use of resonating devices to make small mass measurements. Bull. N. Y. Acad. Med. 1972, 48, 465–467.
- Hammond, D.L.; Benjaminson, A. The crystal resonator- a digital transducer. IEEE Spectr. 1969, 6, 53–58.
- King, W.H.; Camilli, C.T.; Findeis, A.F. Thin Film Thermocouples for Differential Thermal Analysis. Anal. Chem. 1968, 40, 1330–1335.
More
