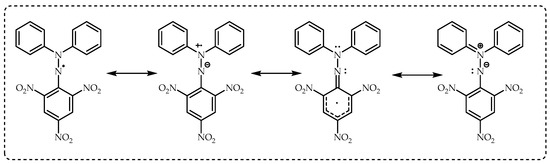
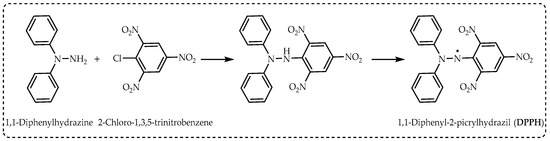
4.1. Evaluation of DPPH Radical Scavenging
DPPH radical scavenging ability is determined mostly in organic solvents such as methanol or ethanol by measuring the absorbance drop at 517 nm
[23][101]. The use of methanol is not preferred because of its toxic properties. Analyses were performed with a UV-vis spectrophotometer in 1 mL or 3 mL cuvettes. For this purpose, a stock solution of 10
−3 M DPPH radicals in ethanol or methanol was freshly prepared before analysis. To prepare the DPPH solution, 3 mL of the stock solution was diluted to 50 mL with methanol in a volumetric flask and protected from light with aluminum foil. Absorbance values were set to 1.00 ± 0.200. Then, 3 mL of DPPH working solution was transferred to the 0.5 mL extract, mixed, and left in the dark for 30 min. The purple color disappears when an antioxidant agent is present in the reaction medium. A reference sample containing 0.5 mL of solvent was similarly prepared. A newly prepared DPPH radical solution shows maximal absorption at 517 nm. All analyses were carried out in 3 replicates, and absorbance was recorded at 517 nm. The blank is the reaction mixture that does not contain test compounds
[12][24][1,102].
4.2. Evaluation of DPPH Radical Scavenging as TEAC
Another way to evaluate the results of DPPH’s radical-removing ability is to express them as Trolox equivalent antioxidant capacity (TEAC). For this purpose, the radical-removing activity (RSA) of Trolox standard solution at different concentrations is determined. In this assay, Trolox, as a standard radical scavenger compound, is interpolated into a dose–response curve. Then, using these values (%RSA)—(Trolox; µM/L), a calibration curve is prepared. For herbal extracts or chemicals prepared at different concentrations, the TEAC (µM/L) was calculated, and Trolox equivalent antioxidant capacities were calculated using the linear regression equation obtained in the linearity range (0.01–0.05 µM/L)
[13][14].
4.3. The Importance of the IC
50
Value in DPPH Radical Scavenging Activity
Different antioxidant concentrations are used to determine the antioxidant concentration that scavenges 50% of the initial DPPH radicals in a specific but arbitrary time interval. This concentration was also referred to as “EC
50”, short for “efficient concentration” or sometimes as “IC
50”, short for “inhibitory concentration”. Indeed, this EC
50 designation has found appropriate scientific use in drug testing under a different name “LD
50”. These terms became widely accepted as “IC
50” to indicate the practicality of antioxidant testing using DPPH radicals. The lower the IC
50 values, the higher the DPPH radical-removing ability of the antioxidants. In this context, the IC
50 value is widely used in biochemistry to compare the radical scavenging capacities of different antioxidants
[25][103]. The IC
50 values quantitatively describe the radical scavenging affinity. For all these reasons, the IC
50 value is one of the most practical ways to evaluate DPPH radical scavenging affinities. The radical scavenging activity (RSA) of maca extracts was calculated using the following equation:
where A
c is the absorbance at 517 nm of the control sample, and A
s is the absorbance at 517 nm that contains the test sample, including plant extracts or pure compounds. The IC
50 was calculated from the graph plotting scavenging percentage against test sample concentration (µg/mL). DPPH radicals decrease significantly upon exposure to radical remover
[26][104].
4.4. Scope of DPPH Radical Scavenging Applications
DPPH radical scavenging is a popular spectrophotometric method that has a wide application area and is used for determining the antioxidant capacity of beverages, pure substances, foods, and herbal extracts. This method is simple, sensitive, fast, and reproducible, making it the most convenient and common radical removal method for evaluating the antioxidant capacity of compounds and herbal extracts.
5. Limitations of the DPPH Assay
In DPPH removal activity, it is very important to convert the moles of DPPH lost by using the change in absorbance, Beer’s law, and damping ε values of 10,900–12,500. Another important point is that this method does not detect reaction rates and ignores some crucial information in the reaction curves
[27][160]. The reactions of DPPH and antioxidants appear to be complex when changes in absorbance are continuously monitored. Although the reaction curves were similar to ABTS
•+ removal, there were some differences when comparing the studied phenolic compounds
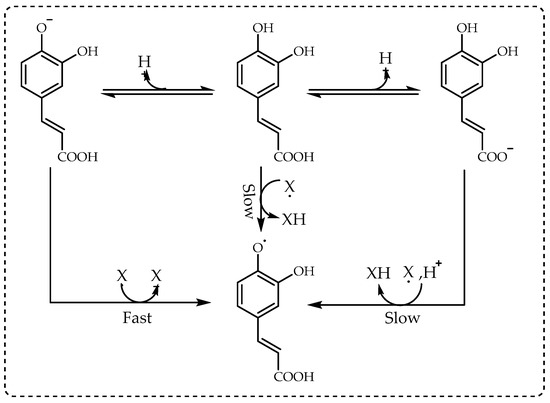
[8][85]. In DPPH radical scavenging, antioxidants can react with DPPH radicals by very fast electron transfer and slow hydrogen atom transfer. Although electron transfer is quite fast, it is slower than ABTS
•+’s reactions with antioxidants due to the difficult accessibility of phenolic compounds to the radical site of the DPPH molecule. This barrier of access inhibits all reactions, particularly the hydrogen transfer, which is necessary for the formation of a hydrogen-bonded complex between the α-C—H radical and the N lone pair required
[28][29][161,162]. Complex molecules get in the way of each other more easily and block access to DPPH radicals at low concentrations and strongly block the reaction at high concentrations. Additionally, methyl alcohol, which is generally used as a solvent for the DPPH method, strongly binds H atoms and inhibits HAT processes
[30][31][32][163,164,165]. However, when water is added to the reaction, it disrupts the bonding and facilitates hydrogen atom transfer. Any test compound with H-atom transfer capability will increase the reaction rate. In the same manner, electron transfer is pH-dependent, with speed increasing with pH and ionization degree, while HAT is pH-independent. The dominant mechanism of a test compound can be evaluated by reacting it with DPPH radicals in methanol and 50% methanol, where the water phase is buffered to a pH range from acidic to alkaline
[8][33][34][85,166,167].