Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by BEIWEN YING.
Genome reduction is a top-down approach to achieve the minimal genetic information essential for a living cell. Exploring the minimal genetic requirements for cells to maintain free living is an exciting topic in biology. Multiple approaches are employed to address the question of the minimal genome. In addition to constructing the synthetic genome in the test tube, reducing the size of the wild-type genome is a practical approach for obtaining the essential genomic sequence for living cells. The well-studied Escherichia coli has been used as a model organism for genome reduction owing to its fast growth and easy manipulation.
- genome reduction
- growth fitness
- minimal genome
- experimental evolution
- genetic requirement
1. Introduction
The genome is the blueprint of life, including all the living organism’s information. The abundance of genetic information a genome contains is nearly determined by the number of base pairs encoded on the genome, both the genes and the non-coding regions. The quantitative evaluation of the genetic abundance is known as the genome size, which largely varies among organisms. In general, the more complex the structure and ecology of an organism, the larger its genome size. For example, a typical virus has a tiny genome size of about 1~30 kbp [1], while a human cell has a genome of 6.4 Gb as diploid, and one of the ferns, Paris japonica, has a giant genome of 597 Gb as octaploid [2,3][2][3]. It is known that the genome size range in archaea and bacteria is between 100 kbp and 16 Mb, which can be linked to the microorganism’s ecosystem type (aquatic, host-associated, or terrestrial) [4]. The genome size roughly represents the abundance of the genetic information required for its holder cell.
Living cells are maintained by transcribing genes encoded in the genome into mRNA, followed by their translation into proteins, which shape cells and catalyse various chemical reactions. The relationship between the genome and the phenotype, the growth fitness, has often been investigated. In addition, genomes have reached their current form through mutations and horizontal gene transfer during evolution; thus, it is crucial to examine genomes to unravel the evolution of organisms. The genome size is the consequence of evolution, which either increases or decreases the genomic sequences. What the minimal genetic information needed is for life on Earth remains an open question.
2. Genome Reduction
Escherichia coli (E. coli) has been widely used in synthetic biology due to its fast growth in rich media [5] and high transformation and plasmid integration efficiency [6]. Nevertheless, the essential and substantial genetic requirements of E. coli are not fully understood. Although E. coli has been well studied in genetics and bioengineering, and its first genome sequence was determined approximately 25 years ago [7], the molecular and physiological functions across the genome have not been fully clarified. Most E. coli genomes range from 3.8 Mb to more than 6 Mb, and the average is around 5 Mb. E. coli K-12 MG1655, one of the most well-known E. coli strains, has about 4.6 Mb and contains approximately 4400 genes, highly interacting with each other and shaping various biological networks. Gene regulatory network (GRN) and transcriptional regulatory network (TRN) were constructed to know the regulatory relationship of genes and often reconstructed to improve the prediction of gene expression [8,9,10,11][8][9][10][11]. Connecting GRN to the metabolic network, which is consisted of metabolites, was implemented to understand the cell system [12]. It is also indicated current GRN may not be able to predict gene expression [13]. A number of genes still have unknown functions, and understanding the entire cell as a dynamic system is challenging due to the complexity of genetic and metabolic networks. In other words, the aim of E. coli genomics is to reveal a regulatory network with thousands of elements, but the genome is far too complex for this purpose.
To discover genetic essentiality, reducing the genetic elements has been challenged to a large extent. Significant efforts have been made to construct a minimal genome containing the essential genes for growing under the defined conditions to provide the genetically simplest model. There are two main approaches to constructing a minimal genome: bottom-up and top-down. The bottom-up approach is represented by landmark works that chemically synthesize a genome containing only essential genes and transfer it to the cytoplasm [14,15,16][14][15][16]. The top-down approach involves genetic deletions by removing redundant DNA sequences from the wild-type (full-length) E. coli genomes, known as genome reduction (Figure 1). Such genetic deletion approaches were employed to a great extent to identify the minimal genetic requirement for a bacterial cell.
Figure 1.
Schematic drawing of genome reduction. A reduced genome is constructed by removing redundant genomic sequences from the parent genome (wild-type genome).
So far, the reduced genomes were mainly constructed with the E. coli strains of MG1655 and W3110, two representative full-length genomes of approximately 4.6 Mb. Multiple deletions of genomic sequences were commonly performed with the traditional genetic methodologies, e.g., λ Red recombinase and P1 transduction, which were conducted many years ago [17,18][17][18]. After the CRISPR/Cas9 system was developed and used widely [19[19][20],20], the random deletion method combining CRISPR/Cas9 and transposon also became available to reduce the genome [21]. Comparative genomics and computational approaches are also available for investigating the genome range of reducible [22]. Various genome-reduced E. coli strains have been successfully constructed in laboratories [23[23][24][25][26],24,25,26], and those of extensive deletions (i.e., more than 10% of the parent wild-type genomes are absent) are summarized (Table 1).
Table 1. Representative genome-reduced E. coli strains. The wide-type strains used as the parent genomes for multiple deletions, the resultant reduced genomes, and their growth fitness in different growth media are summarized according to the previous report [27].
| Parent Genome |
Strain Name | Genome Size (Mb) | Reduced Ratio | Growth Medium |
Growth Fitness |
|---|---|---|---|---|---|
| W3110 (4.66 Mb) |
MGF-01 (N28) | 3.6 | 22% | minimal | decreased |
| minimal, amino acids | decreased | ||||
| rich | decreased | ||||
| DGF-298 | 3.0 | 36% | rich | increased | |
| MG1655 (4.64 Mb) |
MDS42 | 4.0 | 14% | minimal | equivalent |
| rich | equivalent | ||||
| minimal, amino acids | decreased | ||||
| MDS69 | 3.7 | 20% | rich | decreased | |
| Δ16 | 3.3 | 30% | minimal | decreased | |
| MS56 | 3.6 | 23% | minimal | increased, decreased | |
| rich | equivalent, decreased |
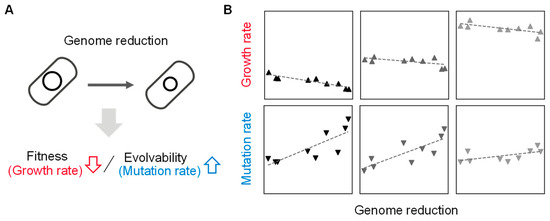
Figure 2. Coordination of genome size to growth and mutation rates. (A) Contribution of genome reduction to fitness and evolvability. The changes in growth and mutation rates caused by the genome reduction are indicated with arrows. (B) Relative values of genome, growth, and mutation. Gray gradation indicates the variation in growth media. The scatter plots are newly made using previously reported data [37,38][37][38]. The panels from left to right represent the nutritional richness of culture media from poor to rich.
3. Evolutionary Approaches for Reduced Genome
In wild nature, free-living bacteria were found to show high growth fitness despite holding small genomes, such as Pelagibacter ubique, an abundant living organism in seawater. It can grow quickly, even with a considerably small genome size of only 20% of that of E. coli (e.g., 1 Mb compared to 5 Mb) [39,40][39][40]. Intriguingly, the Pelagibacter ubique genome contains few transposons or pseudogenes, indicating the reduced genome could have high fitness. It was considered that the living cells had acquired their current fitness through natural evolution. Evolution can be a powerful tool in searching for an essential set of genes to gain improved fitness in the habit (i.e., living environment). Experimental evolution mimics nature evolution under well-controlled laboratory conditions [41,42][41][42]. It is generally conducted through the serial transfer of the bacterial populations to select derivatives with improved fitness. The serial transfer is repeated inoculation and dilution of a portion of the bacterial population grown to the early exponential growth phase into a fresh medium with the same composition (Figure 3A). The end-point (evolved) population finally achieves higher fitness than the initial population (ancestor) (Figure 3B), so it is also called adaptive laboratory evolution (ALE) [43]. The improved fitness of evolved bacterial populations was usually accompanied by the accumulation of genome mutations during evolution. Interestingly, when the experimental evolution of an identical ancestor was performed with multiple lineages under the same condition in parallel, the genome mutations found in the final evolved populations often differed among the lineages [44,45][44][45]. The genomic location of the mutations and the timing of the mutations fixing on the genome varied among the parallelly evolved lineages [46,47][46][47]. It indicates that the experimental evolution provides multiple trajectories for the ancestral genome to acquire somehow finetuned genomic sequences promising growth fitness. So far, intensive studies have demonstrated that experimental evolution is a powerful approach to selecting a bacterial population (genotype) with an increased growth rate (relative fitness) [42,48][42][48].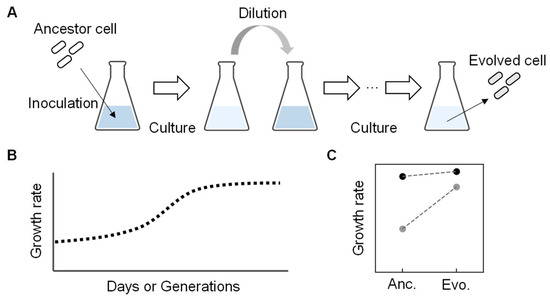
Figure 3. An overview of experimental evolution. (A) Experimental evolution. Repeated culture and dilution are performed with the ancestor to acquire the evolved genome with an improved growth rate. (B) Temporal changes in growth rate during experimental evolution. (C) Growth rate changes between the ancestor and evolved E. coli cells. Dark and light circles represent the full-length and reduced genomes, respectively. The previously reported data [49] were used to make the graph.
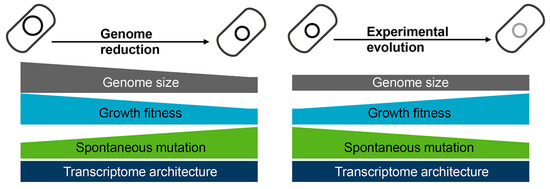
Figure 4. Changes caused by genome reduction and rescued via experimental evolution. Genome reduction causes decreased growth fitness and increased mutation rate (left panel), which is restored via experimental evolution (right panel). The transcriptome architecture maintains homeostasis regardless of genome reduction or experimental evolution.
References
- Campillo-Balderas, J.A.; Lazcano, A.; Becerra, A. Viral genome size distribution does not correlate with the antiquity of the host lineages. Front. Ecol. Evol. 2015, 3, 143.
- Pellicer, J.; Fay, M.F.; Leitch, I.J. The largest eukaryotic genome of them all? Bot. J. Linn. Soc. 2010, 164, 10–15.
- Leitch, I.J.; Pellicer, J.; Hidalgo, O.; Bennett, M.D. Plant DNA C-values Database (Release 7.1). New Phytol. 2019.
- Rodríguez-Gijón, A.; Nuy, J.K.; Mehrshad, M.; Buck, M.; Schulz, F.; Woyke, T.; Garcia, S.L. A Genomic Perspective Across Earth’s Microbiomes Reveals That Genome Size in Archaea and Bacteria Is Linked to Ecosystem Type and Trophic Strategy. Front. Microbiol. 2021, 12, 761869.
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749.
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580.
- Blattner, F.R.; Plunkett, G., III; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The Complete Genome Sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462.
- Martínez-Antonio, A.; Janga, S.C.; Thieffry, D. Functional organisation of Escherichia coli transcriptional regulatory network. J. Mol. Biol. 2008, 381, 238–247.
- Fang, X.; Sastry, A.; Mih, N.; Kim, D.; Tan, J.; Yurkovich, J.T.; Lloyd, C.J.; Gao, Y.; Yang, L.; Palsson, B.O. Global transcriptional regulatory network for Escherichia coli robustly connects gene expression to transcription factor activities. Proc. Natl. Acad. Sci. USA 2017, 114, 10286–10291.
- Fu, Y.; Jarboe, L.R.; Dickerson, J.A. Reconstructing genome-wide regulatory network of E. coli using transcriptome data and predicted transcription factor activities. BMC Bioinform. 2011, 12, 233.
- Al-Aamri, A.; Kudlicki, A.S.; Maalouf, M.; Taha, K.; Homouz, D. Inferring Gene Regulatory Networks from RNA-seq Data Using Kernel Classification. Biology 2023, 12, 518.
- Kumar, S.; Mahajan, S.; Jain, S. Feedbacks from the metabolic network to the genetic network reveal regulatory modules in E. coli and B. subtilis. PLoS ONE 2018, 13, e0203311.
- Larsen, S.J.; Röttger, R.; Schmidt, H.H.H.W.; Baumbach, J. E. coli gene regulatory networks are inconsistent with gene expression data. Nucleic Acids Res. 2019, 47, 85–92.
- Hutchison, C.A., 3rd; Chuang, R.-Y.; Noskov, V.N.; Assad-Garcia, N.; Deerinck, T.J.; Ellisman, M.H.; Gill, J.; Kannan, K.; Karas, B.J.; Ma, L.; et al. Design and synthesis of a minimal bacterial genome. Science 2016, 351, aad6253.
- Gibson, D.G.; Glass, J.I.; Lartigue, C.; Noskov, V.N.; Chuang, R.-Y.; Algire, M.A.; Benders, G.A.; Montague, M.G.; Ma, L.; Moodie, M.M.; et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 2010, 329, 52–56.
- Gibson, D.G.; Benders, G.A.; Andrews-Pfannkoch, C.; Denisova, E.A.; Baden-Tillson, H.; Zaveri, J.; Stockwell, T.B.; Brownley, A.; Thomas, D.W.; Algire, M.A.; et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science 2008, 319, 1215–1220.
- Posfai, G.; Kolisnychenko, V.; Bereczki, Z.; Blattner, F.R. Markerless gene replacement in Escherichia coli stimulated by a double-strand break in the chromosome. Nucleic Acids Res. 1999, 27, 4409–4415.
- Kato, J.-I.; Hashimoto, M. Construction of long chromosomal deletion mutants of Escherichia coli and minimization of the genome. Methods Mol. Biol. 2008, 416, 279–293.
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821.
- Li, Y.; Lin, Z.; Huang, C.; Zhang, Y.; Wang, Z.; Tang, Y.-J.; Chen, T.; Zhao, X. Metabolic engineering of Escherichia coli using CRISPR–Cas9 meditated genome editing. Metab. Eng. 2015, 31, 13–21.
- Ma, S.; Su, T.; Liu, J.; Lu, X.; Qi, Q. Reduction of the Bacterial Genome by Transposon-Mediated Random Deletion. ACS Synth. Biol. 2022, 11, 668–677.
- LeBlanc, N.; Charles, T.C. Bacterial genome reductions: Tools, applications, and challenges. Front. Genome Ed. 2022, 4, 957289.
- Karcagi, I.; Draskovits, G.; Umenhoffer, K.; Fekete, G.; Kovács, K.; Méhi, O.; Balikó, G.; Szappanos, B.; Györfy, Z.; Fehér, T.; et al. Indispensability of Horizontally Transferred Genes and Its Impact on Bacterial Genome Streamlining. Mol. Biol. Evol. 2016, 33, 1257–1269.
- Posfai, G.; Plunkett, G., 3rd; Fehér, T.; Frisch, D.; Keil, G.M.; Umenhoffer, K.; Kolisnychenko, V.; Stahl, B.; Sharma, S.S.; de Arruda, M.; et al. Emergent properties of reduced-genome Escherichia coli. Science 2006, 312, 1044–1046.
- Kato, J.; Hashimoto, M. Construction of consecutive deletions of the Escherichia coli chromosome. Mol. Syst. Biol. 2007, 3, 132.
- Kotaka, Y.; Hashimoto, M.; Lee, K.-I.; Kato, J.-I. Mutations identified in engineered Escherichia coli with a reduced genome. Front. Microbiol. 2023, 14, 1189877.
- Kurokawa, M.; Ying, B.-W. Experimental Challenges for Reduced Genomes: The Cell Model Escherichia coli. Microorganisms 2019, 8, 3.
- Mori, H.; Mizoguchi, H.; Fujio, T. Escherichia coli minimum genome factory. Biotechnol. Appl. Biochem. 2007, 46, 157–167.
- Mizoguchi, H.; Sawano, Y.; Kato, J.-I.; Mori, H. Superpositioning of Deletions Promotes Growth of Escherichia coli with a Reduced Genome. DNA Res. 2008, 15, 277–284.
- Ran, H.; Wu, J.; Wu, D.; Duan, X. Enhanced Production of Recombinant Thermobifida fusca Isoamylase in Escherichia coli MDS42. Appl. Biochem. Biotechnol. 2016, 180, 464–476.
- Lee, J.H.; Sung, B.H.; Kim, M.S.; Blattner, F.R.; Yoon, B.H.; Kim, J.H.; Kim, S.C. Metabolic engineering of a reduced-genome strain of Escherichia coli for L-threonine production. Microb. Cell Factories 2009, 8, 2.
- Reuß, D.R.; Altenbuchner, J.; Mäder, U.; Rath, H.; Ischebeck, T.; Sappa, P.K.; Thürmer, A.; Guérin, C.; Nicolas, P.; Steil, L.; et al. Large-scale reduction of the Bacillus subtilis genome: Consequences for the transcriptional network, resource allocation, and metabolism. Genome Res. 2017, 27, 289–299.
- Suárez, R.A.; Stülke, J.; van Dijl, J.M. Less Is More: Toward a Genome-Reduced Bacillus Cell Factory for “Difficult Proteins”. ACS Synth. Biol. 2019, 8, 99–108.
- Zhang, F.; Huo, K.; Song, X.; Quan, Y.; Wang, S.; Zhang, Z.; Gao, W.; Yang, C. Engineering of a genome-reduced strain Bacillus amyloliquefaciens for enhancing surfactin production. Microb. Cell Factories 2020, 19, 223.
- Kurokawa, M.; Seno, S.; Matsuda, H.; Ying, B.-W. Correlation between genome reduction and bacterial growth. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2016, 23, 517–525.
- Foster, P.L. Methods for Determining Spontaneous Mutation Rates. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2006; Volume 409, pp. 195–213.
- Nishimura, I.; Kurokawa, M.; Liu, L.; Ying, B.-W. Coordinated Changes in Mutation and Growth Rates Induced by Genome Reduction. MBio 2017, 8, e00676-17.
- Lao, Z.; Matsui, Y.; Ijichi, S.; Ying, B.-W. Global coordination of the mutation and growth rates across the genetic and nutritional variety in Escherichia coli. Front. Microbiol. 2022, 13, 990969.
- Giovannoni, S.J.; Tripp, H.J.; Givan, S.; Podar, M.; Vergin, K.L.; Baptista, D.; Bibbs, L.; Eads, J.; Richardson, T.H.; Noordewier, M.; et al. Genome streamlining in a cosmopolitan oceanic bacterium. Science 2005, 309, 1242–1245.
- Viklund, J.; Ettema, T.J.; Andersson, S.G.E. Independent Genome Reduction and Phylogenetic Reclassification of the Oceanic SAR11 Clade. Mol. Biol. Evol. 2011, 29, 599–615.
- Bergh, B.V.D.; Swings, T.; Fauvart, M.; Michiels, J. Experimental Design, Population Dynamics, and Diversity in Microbial Experimental Evolution. Microbiol. Mol. Biol. Rev. 2018, 82, e00008-18.
- Kawecki, T.J.; Lenski, R.E.; Ebert, D.; Hollis, B.; Olivieri, I.; Whitlock, M.C. Experimental evolution. Trends Ecol. Evol. 2012, 27, 547–560.
- Dragosits, M.; Mattanovich, D. Adaptive laboratory evolution—Principles and applications for biotechnology. Microb. Cell Factories 2013, 12, 64.
- Lu, H.; Aida, H.; Kurokawa, M.; Chen, F.; Xia, Y.; Xu, J.; Li, K.; Ying, B.-W.; Yomo, T. Primordial mimicry induces morphological change in Escherichia coli. Commun. Biol. 2022, 5, 24.
- Suzuki, S.; Horinouchi, T.; Furusawa, C. Prediction of antibiotic resistance by gene expression profiles. Nat. Commun. 2014, 5, 5792.
- Kishimoto, T.; Ying, B.-W.; Tsuru, S.; Iijima, L.; Suzuki, S.; Hashimoto, T.; Oyake, A.; Kobayashi, H.; Someya, Y.; Narisawa, D.; et al. Molecular Clock of Neutral Mutations in a Fitness-Increasing Evolutionary Process. PLoS Genet. 2015, 11, e1005392.
- Toprak, E.; Veres, A.; Michel, J.-B.; Chait, R.; Hartl, D.L.; Kishony, R. Evolutionary paths to antibiotic resistance under dynamically sustained drug selection. Nat. Genet. 2011, 44, 101–105.
- Lenski, R.E. Experimental evolution and the dynamics of adaptation and genome evolution in microbial populations. ISME J. 2017, 11, 2181–2194.
- Kurokawa, M.; Nishimura, I.; Ying, B.-W. Experimental Evolution Expands the Breadth of Adaptation to an Environmental Gradient Correlated with Genome Reduction. Front. Microbiol. 2022, 13, 826894.
- Csörgő, B.; Fehér, T.; Tímár, E.; Blattner, F.R.; Pósfai, G. Low-mutation-rate, reduced-genome Escherichia coli: An improved host for faithful maintenance of engineered genetic constructs. Microb. Cell Factories 2012, 11, 11.
- Umenhoffer, K.; Fehér, T.; Balikó, G.; Ayaydin, F.; Pósfai, J.; Blattner, F.R.; Pósfai, G. Reduced evolvability of Escherichia coli MDS42, an IS-less cellular chassis for molecular and synthetic biology applications. Microb. Cell Factories 2010, 9, 38.
- Choe, D.; Lee, J.H.; Yoo, M.; Hwang, S.; Sung, B.H.; Cho, S.; Palsson, B.; Kim, S.C.; Cho, B.-K. Adaptive laboratory evolution of a genome-reduced Escherichia coli. Nat. Commun. 2019, 10, 935.
- Matsui, Y.; Nagai, M.; Ying, B.-W. Growth rate-associated transcriptome reorganization in response to genomic, environmental, and evolutionary interruptions. Front. Microbiol. 2023, 14, 1145673.
More
