Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Ulrich Gergs.
Dopamine has effects on the mammalian heart. These effects can include an increase in the force of contraction, and an elevation of the beating rate and the constriction of coronary arteries. Depending on the species studied, positive inotropic effects were strong, very modest, or absent, or even negative inotropic effects occurred.
- dopamine
- dopamine receptors
- dopamine metabolism
- human heart
1. Introduction
Dopamine is a catecholamine (Figure 1). Dopamine can be of exogenous or endogenous origin. Dopamine has been studied for decades, and a wealth of information on dopamine has been accumulated in the literature. Dopamine has many functions of the mammalian body in health and disease. Practically all organs contain dopamine and can synthesize dopamine. Hence, dopamine must subserve a very critical role in all animals, including humans. Most efforts have been put into the role of dopamine in the central nervous system. In the brain, dopamine can alter the mood of the person. Dopamine depletion in the brain occurs when Morbus Parkinson develops. A lot of work has been focused on how to alter this reduction in dopamine in Morbus Parkinson. Dopamine plays another important role(s) in psychiatric diseases: many antipsychotic drugs (neuroleptic drugs) interfere with dopamine action in the brain. Perhaps it is worth mentioning the many side effects of recreational drugs such as lysergic acid diethylamide (LSD) that may involve dopamine receptors in the brain.
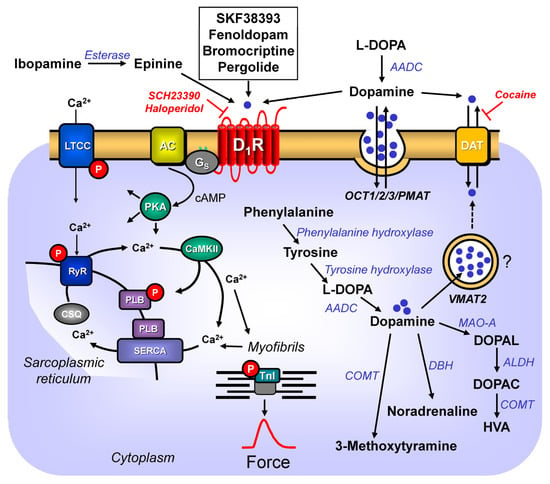
Figure 1.
Dopamine might stimulate D
1
-dopamine receptors in the sarcolemma.
2. Dopamine Synthesis
Dopamine (3,4-dihydroxyphenethylamine or 3-hydroxytyramine) has been called the third endogenous catecholamine, next to adrenaline and noradrenaline (e.g., [16,17][1][2]). Dopamine (earlier, also named oxytyramin [18][3]) is an important endogenous biogenic amine in mammals. Dopamine is thought to play some role in diseases such as schizophrenia and Morbus Parkinson (review: [19][4]). Dopamine has been known since the 1950s to be a neurotransmitter in the central nervous system. In this regard, it is important to keep in mind that dopamine does not pass the blood–brain barrier [20][5]. Dopamine found in the heart or plasma is thus produced in peripheral organs [20][5]. Indeed, outside the brain, dopamine is mainly produced in the cortex of the adrenal gland, and in the gut [20][5]. In the plasma, low levels of dopamine were detected (0.1–0.2 nM), that were suggested to originate from nerve cells [21][6]. In brief, dopamine is built in the human body from the amino acid L-tyrosine (Figure 1) via at least two pathways. L-tyrosine (L-4-hydroxyphenylalanine) itself is formed by hydroxylation in the body from L-phenylalanine, an essential amino acid. A phenylalanine-hydroxylase converts L-phenylalanine to L-tyrosine. In youth, phenylalanine-hydroxylase is solely found in the kidney and liver of mammals [22][7]. However, upon ageing, phenylalanine-hydroxylase is expressed also in cardiomyocytes (mouse, man: [22][7]). Hence, over time, the cardiac production of tyrosine as the precursor of dopamine will increase. The following minor pathway for dopamine generation is known, at least in the brain, and potentially in the heart: L-tyrosine can be first decarboxylated by a decarboxylase to tyramine (4-hydroxyphenyl-ethylamine), and then tyramine is hydroxylated by cytochrome 2D6 [23][8]. Interestingly, cytochrome 2D6 is also expressed in the human heart [24][9]. Hence, dopamine might also be formed from tyramine in the human heart. This cardiac pathway has apparently not yet been reported in humans. One would predict that medicinal drugs that inhibit the activity of cytochrome 2D6 (review of inhibitory drugs: [25][10]) should, in part, reduce the generation, and thus the levels of dopamine in the heart, as they would inhibit this alternative pathway. In the main pathway (Figure 1), tyrosine is converted by the enzyme tyrosine-hydroxylase (Figure 1) to DOPA (L-3,4-dihydroxyphenylalamine, L-DOPA, Levo-DOPA). This hydroxylation is the rate-limiting step in the dopamine-synthesis (review: [20][5]). This tyrosine-hydroxylase is present in the heart [26,27][11][12]. Tyrosine-hydroxylase is phosphorylated, and thereby activated by means of a cAMP-dependent protein kinase [28][13]. Isoprenaline, a β-adrenoceptor agonist and thus a cAMP-increasing agent, increased the phosphorylation state of tyrosine-hydroxylase in isolated rat ganglia [29][14]. Thus, it would be informative to study whether isoprenaline might increase the phosphorylation state of tyrosine-hydroxylase in the human heart. This has apparently not yet been reported. Should that be the case, there might be a way for how β-adrenergic stimulation could rapidly increase dopamine levels and thus amplify the role of dopamine in the heart. In principle, the activity of tyrosine-hydroxylase can vary regionally in the heart: an increased activity of tyrosine-hydroxylase was noted in the canine sinus node, compared to the canine working myocardium. This might indicate an important, but still not understood, role of dopamine for the initiation of the heartbeat [30,31][15][16]. After the constitutive deletion of tyrosine-hydroxylase in mouse, less DOPA and dopamine were formed in the body, as would be expected for a rate-limiting enzyme [32][17]. When tyrosine-hydroxylase was knocked down selectively in the sympathorenal system, the dopamine levels and the noradrenaline levels in the mouse heart decreased [33][18]. This indicates that some of the dopamine found in the heart originates from sympathetic neurons [33][18]. DOPA loses its acid structure via the activity of a decarboxylase (Figure 1), and thus, dopamine is formed [31][16]. The enzyme decarboxylase is also found in the heart [31][16]. Hence, the decarboxylation from dopamine might take place in the heart.
3. Dopamine Levels and Metabolism in the Heart
Convincing data support the presence of dopamine in the mammalian heart. These include the canine left ventricle (40.9 ng/g; 0.24 µM [34][19]), in the heart of rats (468 ng/g; 2.81 µM, [35][20]), or in the septum of the human heart (100 ng/g: 0.67 µM). Even after orthotopic cardiac transplantation, dopamine was detected, albeit in lower concentrations, in the human heart (25 ng/g: 0.17 µM [36][21]). In mouse heart, one reported similar dopamine concentrations, namely, 0.7 pg/mg protein (0.47 µM [33][18]). In the rat atrium, about 20 ng/g of dopamine, in contrast to dopamine levels of about 1 ng/g, in the rat ventricle were reported, indicating a regional difference [37][22]. These concentrations of dopamine are, at least in the organ bath, high enough to exert positive inotropic effects in isolated human cardiac preparations [38][23]. Thus, dopamine concentrations present in the heart might be relevant to sustaining cardiac contractility in humans. As mentioned before, all dopamine-synthetizing enzymes are present in the heart, and possibly even in cardiomyocytes. Therefore, it is conceivable that dopamine is not only present in the heart, but even produced in cardiomyocytes [20][5]. However, this issue needs further experimental studies. Any role of dopamine within the cardiomyocyte is speculative: dopamine might be a metabolic intermediate or a pre-drug for noradrenaline (Figure 1); possibly, dopamine serves autocrine and paracrine purposes: dopamine might stimulate dopamine receptors on cells such as cardiomyocytes, cardiac endothelial cells, or cardiac smooth muscle cells [39][24].
How is dopamine then degraded? Dopamine can be converted by a dopamine-β-hydroxylase that is also expressed in the heart [26][11] to noradrenaline (Figure 1). In this way, dopamine would act as a pro-drug of noradrenaline. When one constitutively deleted dopamine-β-hydroxylase, no noradrenaline was formed, indicating that dopamine-β-hydroxylase is the rate-limiting enzyme for the formation of noradrenaline in general [40][25]. It would be interesting to know how the noradrenaline level in the heart would change if the heart- or even cardiomyocyte-specific deletion of dopamine-β-hydroxylase were performed. If the cardiac production of noradrenaline from dopamine were relevant, one might predict then that little noradrenaline would be present in the heart, but normal levels of noradrenaline in all other tissue. Moreover, dopamine is degraded by means of catecholamine-O-methyltransferase (COMT) (Figure 1) to 3-methoxytyramine. In addition, dopamine in the mitochondria is oxidized by means of monoamine oxidases (MAO), and thus, it can also be converted to (Figure 1) 3-methoxytyramine [20][5]: consistent with this scheme, after the genetic deletion of COMT or MAO, the dopamine levels in the organs of mice increased [41,42][26][27].
Extracellular dopamine can reach the cytosol of a cell via a dopamine-transporter (DAT) (Figure 1), which is also found in the heart [43][28]. At least within the cell bodies of nerve cells, noradrenaline and dopamine can be transported by a vesicular monoamine-transporter (VMAT2) (Figure 1) into storage vesicles, where dopamine is protected from enzymes that might degrade dopamine [20,43][5][28]. In addition, this dopamine-transporter (VMAT2) has been detected in mammalian hearts [43][28]. A role of VMAT2 in cardiomyocytes needs to be better defined: cardiomyocytes do not contain storage vesicles for monoamines. Therefore, it might be worthwhile to study whether or not VMAT2 is expressed on the mRNA- or on the protein-level in cardiomyocytes. In the kidney, sufficient dopamine is produced locally that these renally formed dopamine levels are high enough to stimulate renal dopamine receptors. Indeed, hypertension might result in some patients from their inability to produce enough dopamine in their kidneys (review [44][29]).
References
- Brodde, O.E. Brodde, O.-E. Physiology and pharmacology of cardiovascular catecholamine receptors: Implications for treatment of chronic heart failure. Am. Heart J. 1990, 120, 1565–1572.. Am. Heart J. 1990, 120, 1565, 10.1016/0002-8703(90)90060-B.Brodde, O.-E. Physiology and pharmacology of cardiovascular catecholamine receptors: Implications for treatment of chronic heart failure. Am. Heart J. 1990, 120, 1565–1572.
- Brodde, O.E. Dopamine, cardiovascular dopamine receptors and chronic heart failure.. Cardiologia 1995, 40, 125.Brodde, O.E. Dopamine, cardiovascular dopamine receptors and chronic heart failure. Cardiologia 1995, 40 (Suppl. S1), 125–129.
- Holtz, P.; Credner, K.; Koepp, W. Die enzymatische Entstehung von Oxytyramin im Organismus und die physiologische Bedeutung der Dopadecarboxylase. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1942, 200, 356–388.
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol. Ther. 2019, 203, 107392.
- Sonne, J.; Lopez-Ojeda, W. Dopamine; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535451/ (accessed on 10 December 2022).
- Goldstein, D.S.; Holmes, C. Neuronal source of plasma dopamine. Clin. Chem. 2008, 54, 1864–1871.
- Czibik, G.; Mezdari, Z.; Altintas, D.M.; Bréhat, J.; Pini, M.; D’Humières, T.; Delmont, T.; Radu, C.; Breau, M.; Liang, H.; et al. Dysregulated Phenylalanine Catabolism Plays a Key Role in the Trajectory of Cardiac Aging. Circulation 2021, 144, 559–574.
- Niwa, T.; Sugimoto, S. Inhibitory and Stimulatory Effects of Selective Serotonin Reuptake Inhibitors on Cytochrome P450 2D6-mediated Dopamine Formation from p-Tyramine. J. Pharm. Pharm. Sci. 2019, 22, 585–592.
- Thum, T.; Borlak, J. Gene expression in distinct regions of the heart. Lancet 2000, 355, 979–983.
- Cicali, E.J.; Smith, D.M.; Duong, B.Q.; Kovar, L.; Cavallari, L.H.; Johnson, J.A. A Scoping Review of the Evidence Behind Cytochrome P450 2D6 Isoenzyme Inhibitor Classifications. Clin. Pharmacol. Ther. 2020, 108, 116–125.
- Gavrilovic, L.; Spasojevic, N.; Zivkovic, M.; Dronjak, S. Effect of immobilization stress on gene expression of catecholamine biosynthetic enzymes in heart auricles of socially isolated rats. Braz. J. Med. Biol. Res. 2009, 42, 1185–1190.
- Li, Y.; Li, J.; Tang, B.-P.; Gan, T.; Xu, G.; Zhou, X.; Li, H.; Guo, X.; Mahemuti, A.; Sun, Q.; et al. Expression of tyrosine hydroxylase and growth-associated protein 43 in aging atrial fibrillation patients of Xinjiang Uygur and Han nationality. Genet. Mol. Res. 2013, 12, 5257–5266.
- Dunkley, P.R.; Dickson, P.W. Tyrosine hydroxylase phosphorylation in vivo. J. Neurochem. 2019, 149, 706–728.
- Cahill, A.L.; Perlman, R.L. Phosphorylation of tyrosine hydroxylase in the superior cervical ganglion. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 1984, 805, 217–226.
- Endoh, M. Effects of dopamine on sinus rate and ventricular contractile force of the dog heart in vitro and in vivo. Br. J. Pharmacol. 1975, 55, 475–486.
- Dickson, D.W.; Lund, D.D.; Subieta, A.R.; Prall, J.L.; Schmid, P.G.; Roskoski, R., Jr. Regional distribution of tyrosine hydroxylase and dopamine beta-hydroxylase activities in guinea pig heart. J. Auton. Nerv. Syst. 1981, 4, 319–326.
- Locke, T.M.; Fujita, H.; Hunker, A.; Johanson, S.S.; Darvas, M.; du Lac, S.; Zweifel, L.S.; Carlson, E.S. Purkinje Cell-Specific Knockout of Tyrosine Hydroxylase Impairs Cognitive Behaviors. Front. Cell. Neurosci. 2020, 14, 228.
- Miyajima, K.; Kawamoto, C.; Hara, S.; Mori-Kojima, M.; Ohye, T.; Sumi-Ichinose, C.; Saito, N.; Sasaoka, T.; Metzger, D.; Ichinose, H. Tyrosine hydroxylase conditional KO mice reveal peripheral tissue-dependent differences in dopamine biosynthetic pathways. J. Biol. Chem. 2021, 296, 100544.
- Soares-Da-Silva, P.; Davidson, R. Effects of 6-hydroxydopamine on dopamine and noradrenaline content in dog blood vessels and heart. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1985, 329, 253–257.
- Graham, J.D.; Lewis, M.J.; Williams, J. Proceedings: The effect of delta-1-tetrahydrocannabinol on the noradrenaline and dopamine content of the brain and heart of the rat. Br. J. Pharmacol. 1974, 52, 446P.
- Regitz, V.; Bossaller, C.; Strasser, R.; Schüler, S.; Hetzer, R.; Fleck, E. Myocardial catecholamine content after heart transplantation. Circulation 1990, 82, 620–623.
- Elayan, H.; Kennedy, B.; Ziegler, M.G. Propranolol reduces rat dopamine-β-hydroxylase activity and catecholamine levels. Eur. J. Pharmacol. 1992, 212, 259–262.
- Brodde, O.-E.; Vogelsang, M.; Broede, A.; Michel-Reher, M.; Beisenbusch-Schäfer, E.; Hakim, K.; Zerkowski, H.-R. Diminished Responsiveness of Gs-Coupled Receptors in Severely Failing Human Hearts: No Difference in Dilated Versus Ischemic Cardiomyopathy. J. Cardiovasc. Pharmacol. 1998, 31, 585–594.
- Goodman, L.S.; Gilman, A. The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2018.
- Cubells, J.F.; Schroeder, J.P.; Barrie, E.S.; Manvich, D.F.; Sadee, W.; Berg, T.; Mercer, K.; Stowe, T.A.; Liles, L.C.; Squires, K.E.; et al. Human Bacterial Artificial Chromosome (BAC) Transgenesis Fully Rescues Noradrenergic Function in Dopamine β-Hydroxylase Knockout Mice. PLoS ONE 2016, 11, e0154864.
- Fornai, F.; Chen, K.; Giorgi, F.S.; Gesi, M.; Alessandri, M.G.; Shih, J.C. Striatal dopamine metabolism in monoamine oxidase B-deficient mice: A brain dialysis study. J. Neurochem. 2002, 73, 2434–2440.
- Cuevas, S.; Villar, V.A.; Jose, P.A.; Armando, I. Renal Dopamine Receptors, Oxidative Stress, and Hypertension. Int. J. Mol. Sci. 2013, 14, 17553–17572.
- Palomar, A.R.; Larios, B.N.; De Sánchez, V.C.; Pérez, L.M.; López, F.D.L.C.; Flores, G.; Gómez-Villalobos, M.D.J. Expression and Distribution of Dopamine Transporter in Cardiac Tissues of the Guinea Pig. Neurochem. Res. 2010, 36, 399–405.
- Asghar, M.; Tayebati, S.K.; Lokhandwala, M.F.; Hussain, T. Potential Dopamine-1 Receptor Stimulation in Hypertension Management. Curr. Hypertens. Rep. 2011, 13, 294–302.
More
