You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Luis M Amezcua-Guerra and Version 2 by Lindsay Dong.
C-reactive protein (CRP) is an inflammatory molecule that has demonstrated value as a predictive marker for cardiovascular risk assessment, both independently and in conjunction with other parameters. It has been incorporated into risk assessment algorithms, enhancing risk prediction and guiding therapeutic decisions. Pharmacological interventions with anti-inflammatory properties, such as statins, glucagon-like peptide-1 agonists, and interleukin-1 inhibitors, have shown promising effects in reducing both cardiovascular risks and CRP levels.
- C-reactive protein
- coronary artery disease
- atherosclerotic cardiovascular disease
- inflammation
1. Introduction
Atherosclerotic cardiovascular disease (CVD) remains the leading cause of mortality worldwide. While significant progress has been made in understanding and managing conventional risk factors such as hypertension, diabetes, dyslipidemia, and smoking, CVD continues to pose a significant global health challenge [1]. Risk scoring systems incorporating these conventional risk factors have been developed to predict acute coronary syndromes (ACS) and guide therapeutic interventions. Moreover, dietary and pharmacological interventions targeting cardiovascular risk factors have demonstrated substantial benefits in the prevention and progression of coronary artery disease (CAD) [2]. In addition, public policies addressing tobacco use have contributed to reducing the incidence of ACS and the progression of atherosclerotic CVD [3]. However, it is increasingly recognized that traditional risk algorithms may not fully capture the complexities of individual patients. In recent years, inflammation has emerged as a crucial component in the pathogenesis of CVD, with numerous studies elucidating the intricate relationship between inflammation and CAD [4].
Chronic low-grade inflammation, in particular, has been acknowledged as a contributing factor in the initiation, progression, and complications of various cardiovascular conditions, including atherosclerosis, ACS, and heart failure. Interestingly, a reciprocal causality has been unveiled wherein cardiovascular risk factors exhibit the ability to incite inflammatory responses. Indeed, hypertension, dyslipidemia, smoking, diabetes, and obesity have been attributed to the ability to trigger and sustain inflammatory responses. The secretion of proinflammatory molecules may trigger a cascade of events that culminate in the destabilization of atherosclerotic lesions. This reciprocal interplay between inflammation and the pathogenesis of CAD elucidates a compelling rationale for the comprehensive management of cardiovascular risk factors with interventions that improve cardiometabolic status and decrease hypercoagulability while exerting anti-inflammatory effects [4][5][4,5].
To effectively combat CVD, it is imperative to understand the molecular and cellular processes driving the inflammatory response. This understanding is paving the way for the development of targeted anti-inflammatory interventions and bridging the gap between basic research and clinical cardiology. The shift toward translational cardiology enables the transfer of knowledge from the laboratory bench to the patient’s bedside, with the ultimate goal of achieving precision cardiology. The realization of personalized cardiology, wherein interventions are tailored to individual patients based on their unique clinical, thrombotic, and inflammatory profiles, holds great promise. By incorporating insights from the inflammatory milieu underlying each patient, we can enhance risk prediction and refine therapeutic strategies and patient outcomes [6].
2. Inflammation as a Novel Pathogenic Paradigm in Myocardial Infarction
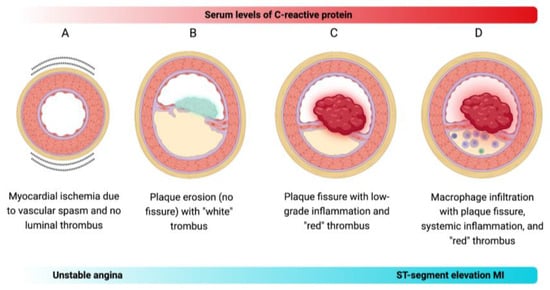
Acute myocardial infarction (MI) triage relies on the electrocardiographic assessment of ST-segment changes, a venerable technology in use for more than a century. In the same way, confirmation of myocardial necrosis is achieved through the measurement of molecules that result from the lysis of cardiomyocytes, such as creatine kinase (CK) and cardiac troponins. Although this strategy has shown to be useful when developing evidence-based medicine, the advent of precision medicine has brought with it the need to integrate data at the individual level, encompassing genomics, novel biomarkers, lifestyle factors, and environmental influences [7][8][7,8]. Early postmortem studies proposed that plaque rupture was the main cause of fatal MI. This led to the concept of vulnerable plaques, characterized by a large central lipid core, inflammatory cell abundance, and a thin fibrous cap. It was believed that the weakening of the collagen structure in thin-capped fibroatheroma, caused by inflammatory mechanisms, resulted in coronary atheromata instability. Consequently, efforts were made to develop methods for detecting vulnerable plaques. However, identifying high-risk plaques was proved challenging due to their low predictive value [7]. Although inflammation is a major contributor to atherosclerosis development, it may not be the sole driver of the transition from stable atherosclerosis to acute thrombosis. Evidence shows that approximately half of ACS may occur even when CRP levels are normal [9]. Moreover, up to one-third of ACS cases are caused by plaque erosion, and about one-fifth of ACS events occur without detectable coronary thrombosis, indicating that functional alterations beyond thrombus formation can contribute to the pathogenesis of CAD [10]. Based on the notion that inflammation alone may not account for all transitions to MI, Crea and Libby have contextualized a new pathogenic paradigm on the various mechanisms that may cause ACS [5]. Briefly, there are four distinct mechanisms that can lead to ACS (Figure 1):
Figure 1. Proposed mechanistic models of ACS. (A) Vasospasm-induced ACS: Vasospasm, known to occur in epicardial arteries, can also impact coronary microcirculation, triggering ACS, such as unstable angina, independently of inflammation. (B) Plaque erosion-induced ACS: Plaque erosion is increasingly recognized as a contributor to ACS, particularly the non-ST-segment elevation MI. The thrombi formed over eroded intimal patches exhibit characteristics of platelet-rich structures, termed the “white” thrombus. (C) Non-inflammatory plaque rupture-induced ACS: Some atheromata may experience plaque rupture without exhibiting extensive intimal macrophage collections or elevated circulating CRP levels. This type of plaque rupture leads to the formation of fibrin-rich “red” thrombi. (D) Inflammatory plaque rupture-induced ACS: Plaque rupture has conventionally been considered the primary cause of ACS, especially the ST-segment elevation MI. It often occurs in plaques featuring local inflammation marked by macrophage infiltration and systemic inflammation indicated by elevated CRP levels in the blood.
3. CRP Holds Potential as a Quintessential Marker of Inflammation
Produced primarily by hepatocytes and endothelial cells under the influence of interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF), CRP is a pentameric protein that belongs to the pentraxin superfamily. Its name originates from its ability to be bound to pneumococcal somatic C polysaccharide. CRP exhibits dual roles in inflammation, functioning as both pro-inflammatory and anti-inflammatory molecules [11][12][11,12]. CRP promotes the recognition and elimination of pathogens, as well as the clearance of necrotic and apoptotic debris. In addition to its high affinity for phosphocholine in the membrane of apoptotic debris, CRP activates the complement system through a pathway parallel to that of lectins, acting as an opsonin for phagocytosis (Figure 2) [13][14][13,14].
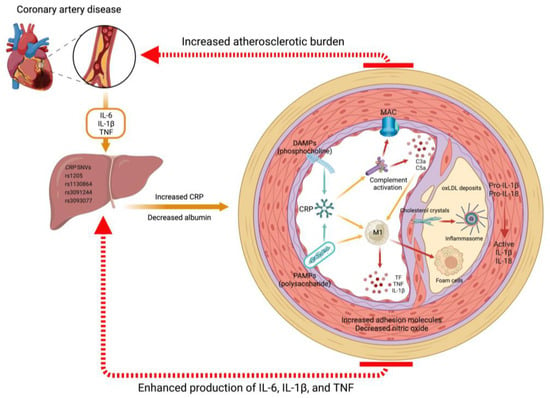

Figure 2. Mechanisms of CRP in atherosclerotic CVD. CRP, predominantly synthesized in the liver under the influence of proinflammatory cytokines such as IL-6, IL-1β, and TNF, plays a pivotal role in the progression of atherosclerotic CVD. Various single nucleotide variants (SNVs) can modulate CRP production. Once released into circulation, CRP exists in a pentameric form and exhibits recognition and binding capabilities to danger-associated molecular patterns (DAMPs), such as the phosphocholine found in apoptotic debris, as well as pathogen-associated molecular patterns (PAMPs), such as the lipopolysaccharide in the membrane of gram-negative bacteria. Upon activation, CRP undergoes a conformational change into functionally active monomers, leading to the initiation of the complement cascade and activation of inflammatory macrophages (M1 phenotype). In the context of chronic inflammation, these stimuli become detrimental and facilitate the deposition of oxidized low-density lipoproteins (oxLDL) and lipid-containing foam cells within the vascular sub-endothelium. The formation of cholesterol crystals in atheromatous plaques further enhances the activation of inflammasomes, which are PAMP and DAMP recognition receptors. Consequently, this process leads to increased production of IL-1β and IL-18. This inflammatory microenvironment triggers heightened production of chemoattractants and adhesion molecules while concomitantly reducing the production of nitric oxide and other vasodilatory factors. Such disruption of vascular endothelial homeostasis results in an augmented atherosclerotic burden and the development of CAD.
Elevated CRP levels are closely associated with both acute and chronic inflammatory conditions, spanning across a broad spectrum of disorders including infection, trauma, stroke, tissular necrosis, and neoplasms [15][18]. The conventional measurement techniques for CRP, commonly utilized to evaluate acute or severe inflammatory responses, often lack the requisite sensitivity to detect the subtle inflammatory nuances characterizing chronic conditions, including CVD. Conversely, the implementation of highly sensitive measurement methodologies enables the detection of high-sensitivity CRP, a refined iteration of the CRP assay. In essence, the principal distinction between CRP and high-sensitivity CRP resides in their inherent sensitivity and the expanse of clinical scenarios wherein they find application. In clinical practice, several techniques are used to measure CRP levels. The choice of a particular technique depends on factors such as the level of sensitivity required, the available resources, and the specific goals of the measurement. Immunonephelometry operates on the formation of antigen-antibody complexes and quantifies the intensity of scattered light when a laser passes through the sample. Enzyme-linked immunosorbent assay is a versatile technique that involves the use of antibodies that are bound to CRP, followed by a colorimetric reaction that quantifies the amount of CRP present. Color-flow cytometry employs fluorescently labeled antibodies to detect and quantify CRP levels within the analyzed cells or serum/plasma samples. Latex agglutination involves mixing CRP-specific antibodies with a latex suspension; in the presence of CRP in the sample, the latex particles agglutinate, allowing for visual detection or assessment through turbidity measurements. Point-of-care tests represent rapid diagnostic tools designed for immediate on-site CRP measurement.
4. Ethnic and Gender Differences in CRP Levels
Cardiovascular risk assessment tools are primarily based on studies consisting predominantly of Caucasian men, which may generate inaccuracies when calculating the cardiovascular risk for populations with different genetics or ethnic-racial backgrounds, or for women.
Khera and colleagues investigated the differences in CRP levels based on ethnic-racial background and gender. Theis study involved 2749 participants, categorized into four groups: Afro-American women, Afro-American men, Caucasian women, and Caucasian men. The findings revealed that Afro-American subjects had higher CRP levels compared to Caucasians (3.0 mg/L vs. 2.3 mg/L; p < 0.001), while women had higher levels than men (3.3 mg/L vs. 1.8 mg/L; p < 0.001). CRP levels > 3 mg/L were found in 31% of Caucasian men, 40% of Afro-American men, 51% of Caucasian women, and 58% of Afro-American women (p < 0.05 for each group vs. Caucasian men). Adjusting for conventional cardiovascular risk factors, estrogen use, statins, and body mass index, the prevalence of CRP levels > 3 mg/L remained significantly higher in Caucasian women (odds ratio [OR], 1.6; 95% confidence interval [CI], 1.1–2.5) and Afro-American women (OR, 1.7; 95% CI, 1.2–2.6), but not in Afro-American men (OR, 1.3; 95% CI, 0.8–1.9) compared to Caucasian men. This highlights the tendency of the populations different from Caucasian men (reference group) to exhibit elevated CRP levels [16][25].
