Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alexandre Almeida and Version 2 by Camila Xu.
Lymphedema is characterized by an abnormal accumulation of protein-rich fluid within the interstitium, resulting in swelling of the affected area. It can manifest as primary lymphedema when it results from a structural or developmental defect in the lymphatic system, or as secondary lymphedema, which is due to iatrogenic causes.
- lymphedema
- preoperative assessment
- surgical treatment
- liposuction
- direct excision
1. Introduction
Lymphedema is characterized by an abnormal accumulation of protein-rich fluid within the interstitium, resulting in swelling of the affected area. It can manifest as primary lymphedema when it results from a structural or developmental defect in the lymphatic system, or as secondary lymphedema, which is due to iatrogenic causes. Most cases of lymphedema in developed countries are secondary, resulting from damage to the lymphatic system induced by cancer or cancer treatment [1][2][1,2]. The diagnosis of lymphedema is primarily clinical and may be confirmed by imaging studies. Nonsurgical treatment remains the cornerstone of early-stage management, with the aim of increasing interstitial pressure and decreasing capillary filtration, preventing the progression to clinical lymphedema. There is also some evidence that conservative treatment, which is widely accepted as the universal first-line therapy for extremity lymphedema, provides benefits in volume reduction for mild lymphedema. However, this approach does not address the underlying lymphatic dysfunction or pathophysiology of disease progression [3]. In cases where nonsurgical management is no longer effective, surgical options are considered, including debulking and physiological procedures. As our ability to understand the pathogenesis of the disease has increased, along with advances in microsurgical techniques, new physiological procedures have been developed, with the goal of restoring lymphatic function and flow within the affected area. These procedures include lymphaticovenous anastomosis and vascularized lymph node transfers. When the disease continues to progress, irreversible damage to the tissue occurs, including extensive fibrosis and the accumulation of adipose tissue, which is a condition that can only be managed via debulking procedures. The goal of those procedures is to reduce limb volume and improve the patient’s symptoms and discomfort, but without the ability to restore lymphatic flow. Although these procedures have shown significant benefits for lymphedema patients, there is a lack of clear guidelines and reproducible studies [2][3][4][5][6][2,3,4,5,6].
2. Imaging for Diagnosis
A clinical evaluation is the primary method of diagnosing lymphedema, and during the physical exam, it is important to exclude other conditions that can cause limb swelling like congestive heart failure, renal failure, malignancy, thyroid disease, and more. Lymphatic imaging studies, such as lymphoscintigraphy and indocyanine green lymphography, can assist in the accurate differential diagnosis of lymphedema, disease staging, and choosing the appropriate treatment modality.2.1. Lymphoscintigraphy
Lymphoscintigraphy (LSG) gives a general overview of the lymphatic function and has been considered the gold standard for confirming lymphedema diagnosis [7]. This technique involves subdermally injecting a technetium-labeled colloid in the distal limb, followed by nuclear scanning to assess the lymphatic system. In patients with lymphedema, colloid transport through the lymphatics to the nodal basin is often compromised, resulting in delayed uptake and fluid leakage into the subcutaneous tissue [8]. Although LSG is helpful in evaluating central lymphatic system abnormalities and the extent of the disease, it has several limitations, including a poor anatomic/spatial resolution, the inability to assess interstitial tissues and accompany the vasculature of the lymphatic system, radiation exposure, and lengthy examination times [1][2][7][1,2,7] (Figure 1).
Figure 1.
Lymphoscintigraphy of the upper limb. It depicts severe left lymphedema.
2.2. Indocyanine Green Lymphography
Indocyanine green lymphography (ICG-L) is currently the preferred diagnostic imaging technique for the lymphatic system among surgeons [8]. It has higher sensitivity and specificity for diagnosing lymphedema compared to lymphoscintigraphy [7]. It is also used for the selection of the surgical treatment modality and to give guidance to conservative treatment via manual lymphatic drainage. An intradermal injection of an ICG tracer in the distal limb is followed by the visualization of the lymphatic vessels under a near-infrared camera. It is a minimally invasive, simple, and highly accurate method for assessing lymphatic system status [9]. It enables the evaluation of the functional status of the superficial lymphatic vessels and the determination of their location, collateral lymphatic circulation, and dermal backflow [2][9][10][2,9,10]. A staging system correlating the disease severity with the pattern of dermal backflow on ICG-L was developed by Yamamoto et al. [11]. A linear pattern is considered to be normal, while splash, stardust, and diffuse patterns represent abnormalities with increasing levels of deterioration in lymphatic function (Figure 2). In severe cases of dermal backflow, the lymphatic flow beneath it is masked and cannot be detected by ICG-L. Other limitations include an inability to visualize lymphatic vessels deeper than 1.5–2.0 cm from the skin’s surface and an inability to assess interstitial tissues, the venous system, or the lymph nodes (except during surgery) [1][7][8][10][1,7,8,10]. Furthermore, the evaluation method is operator-dependent.
Figure 2.
Linear pattern demonstrated by ICG lymphography.
3. Imaging for Treatment
After establishing the diagnosis of lymphedema, the next step is choosing the appropriate treatment option, and the possibility of performing a lymphaticovenous anastomosis (LVA) becomes an important aspect of surgical decision making. Functional lymphatic vessels and nearby receiving veins are important requirements for LVA, and therefore, preoperative imaging plays a significant role in substantiating the treatment choice. An ideal imaging study should evaluate the anatomy and function of the lymph nodes and lymphatic vessels, show their course in three dimensions, and display the venous network that will function as an anastomotic acceptor site [12]. In addition to being a diagnostic tool, indocyanine green lymphography (ICG-L) is also part of the preoperative evaluation for lymphatic surgery. Other helpful imaging modalities include magnetic resonance lymphangiography (MRL), single-photon emission computed tomography/computed tomography (SPECT/CT), ultra-high-frequency ultrasound (UHF-US), and photoacoustic (PA) imaging (Table 1).Table 1.
Overview of the imaging modalities and their advantages and limitations in evaluating the lymphatic system.
| Imaging Modalities | Advantages | Limitations |
|---|---|---|
| LSG |
|
|
| ICG-L |
|
|
| MRL |
|
|
| SPECT/CT |
|
|
| UHF-US |
|
|
| PA imaging |
|
|
3.1. Indocyanine Green Lymphography
As discussed before, indocyanine green lymphography can serve as a valuable tool in the outpatient setting for diagnosis and guiding treatment decisions. During LVA surgery, ICG-L facilitates the localization of lymphatic vessels, distinguishes between normal and abnormal drainage pathways, and optimizes the LVA surgical efficacy by assessing the anastomosis patency. These benefits make ICG-L a valuable intraoperative tool for surgeons performing LVA procedures [11].3.2. Magnetic Resonance Lymphangiography (MRL)
Magnetic resonance lymphangiography (MRL) is typically performed by intradermally injecting a gadolinium-based contrast agent in the interdigital web spaces. There is also a possibility of performing MRL without the injection of a contrast agent, but no information is obtained about the functional status of the lymphatics and veins, and it has a lesser spatial resolution when compared to contrast-enhanced MRL [12]. Contrast-enhanced MRL produces high-resolution images of the lymphatic channels, including the number, size, depth, trajectory, and regions of dermal backflow [9]. In fact, MRL has a higher sensitivity to detect lymphatic vessel abnormalities than other imaging modalities such as lymphoscintigraphy (LSG), indocyanine green lymphangiography (ICG-L), and Ultrasound Doppler [9]. As opposed to ICG-L, MRL provides a three-dimensional image of the entire extremity and provides information about the quantity and quality of both the superficial and deep lymphatic systems. It also allows for the visualization of the lymph node basin, the venous system, and the quality of the interstitial fluid [1][2][1,2]. This capacity to identify and map functional lymphatic channels preoperatively makes MRL useful in determining suitable targets for performing lymphovenous anastomosis (LVA) (Figure 3). Studies [9][13][9,13] found that the concordant use of MRL and ICG-L in identifying functional lymphatic vessels correlates with a higher probability of successful LVA. Additionally, MRL can evaluate the composition of a lymphedematous limb, aiding in the determination of appropriate surgical treatment. Patients with normal subcutaneous tissues or fluid-dominant edema may benefit from microsurgical reconstruction, while adipose-dominant edema is usually treated with liposuction, and fibrosclerotic-dominant edema is treated with direct excision [1]. A study performed by Dayan et al. [14] found that even patients within the same International Society of Lymphology class may have different percentages of fat and fluid, which can impact the treatment choice, and demands an appropriate preoperative assessment. However, MRL is an expensive tool compared to ICG-L, it may produce images with venous enhancement due to venous uptake of the contrast agent, it is time-consuming, difficult for patients with claustrophobia, and might be impossible for patients with non-compatible implants [15].
Figure 3. Magnetic resonance lymphangiography provides anatomic delineation and 3D reconstruction images of lymphatics and adjacent veins. Long arrows = lymphatic channels; short arrows = vein; and circle = the crossing of both (the possible place of incision).
3.3. Single-Photon Emission Computed Tomography/Computed Tomography (SPECT/CT)
SPECT/CT is a hybrid imaging modality that combines the planar imaging of SPECT with CT. This technique provides an anatomical localization of radio-activated lymph nodes and provides functional and three-dimensional information about the lymphatic system [16][17][16,17]. SPECT/CT can differentiate between lymphatic vessels and veins, and between tracer uptake in lymph nodes and lymphoceles. It is also capable of identifying dermal backflow from lymphatic vessel leakage and locating appropriate lymphatic vessels for LVA [18][19][18,19]. However, SPECT/CT has some drawbacks including a high cost and exposure to radiation, as well as low resolution for localizing lymphatic channels [17].3.4. Ultra-High-Frequency Ultrasound (UHF-US)
Ultra-high frequency ultrasound (UHF-US) with a frequency range of 48–70 MHz provides an improved resolution compared to conventional ultrasound (15–24 MHz). It enables the accurate, real-time visualization of the lymphatic vessels, even those with diameters smaller than 0.3 mm, and allows operators to distinguish them from the subcutaneous veins or the nerves [20]. Lymphatic vessels are differentiated based on their shape, echogenic texture, color, Doppler collapsibility, convergence, and location [21]. UHF-US can also classify lymphatic vessels into two types: type I (less obstructed lymphatic channels, including normal and ectasis types) and type II (more obstructed lymphatic channels, including contraction and sclerosis types). Differentiating between these types is important for selecting suitable lymphatic vessels for LVA intraoperatively. Over time, the damaged lymphatic vessels become sclerotic and lose their ability to drain lymph fluid effectively; therefore, type I anastomosing lymphatic vessels have a significant advantage in LVA surgery [22]. UHF-US limitations include operator dependency and limited distance reach, as it can obtain images only up to 10 mm deep from the skin’s surface. To detect lymphatic vessels deeper than 10 mm, a transducer with a frequency of 48 MHz (max image depth: 23.5 mm) is recommended [20].3.5. Photoacoustic (PA) or Optoacoustic Imaging
Photoacoustic imaging is a novel technique that utilizes the photoacoustic effect to visualize the lymphatic and vascular systems in three dimensions with a high resolution. This imaging modality, known as PA lymphangiography, involves the absorption of a specific wavelength of light by chromophores such as melanin, hemoglobin, or ICG, leading to thermoelastic expansion and the production of acoustic waves that are detected using an ultrasound transducer [23][24][23,24]. Unlike ICG-L, which also relies on ICG to identify lymphatic vessels, PA imaging can accurately differentiate between veins and lymphatic vessels, asses the three-dimensional relationship between them, and is less influenced by dermal backflow [23]. Consequently, using PA imaging intraoperatively enables the rapid identification of optimal sites for LVA and the assessment of anastomosis patency. In fact, PA imaging fulfills many of the criteria for an ideal imaging modality for surgical planning. However, it is still a developing technology, and handheld PA imaging devices are limited to a depth of 1 cm. Additionally, accurately adjusting the positions of the PA lymphangiography figures and the patient’s limb for clinical application remains challenging [23][24][23,24]. Despite these challenges, PA lymphangiography shows great promise as a valuable tool in lymphatic surgery.4. Surgical Treatment
Complex decongestive therapy has been widely accepted as a conservative treatment strategy for lymphedema. In cases where it is no longer effective, surgical intervention may be considered. The surgical options can be classified as debulking or physiologic procedures. The former is typically performed in the later stages of the disease when there has been a transition from fluid-dominated edema to adipose or fibrosclerotic-dominated edema. Commonly used debulking procedures include suction-assisted lipectomy and direct excision, both focused on reducing the volume of the limb rather than restoring lymphatic flow. Physiologic treatment involves microsurgery techniques and focuses on restoring lymphatic flow and function. A treatment algorithm for patients with symptoms of lymphedema is provided in Figure 4Figure S1.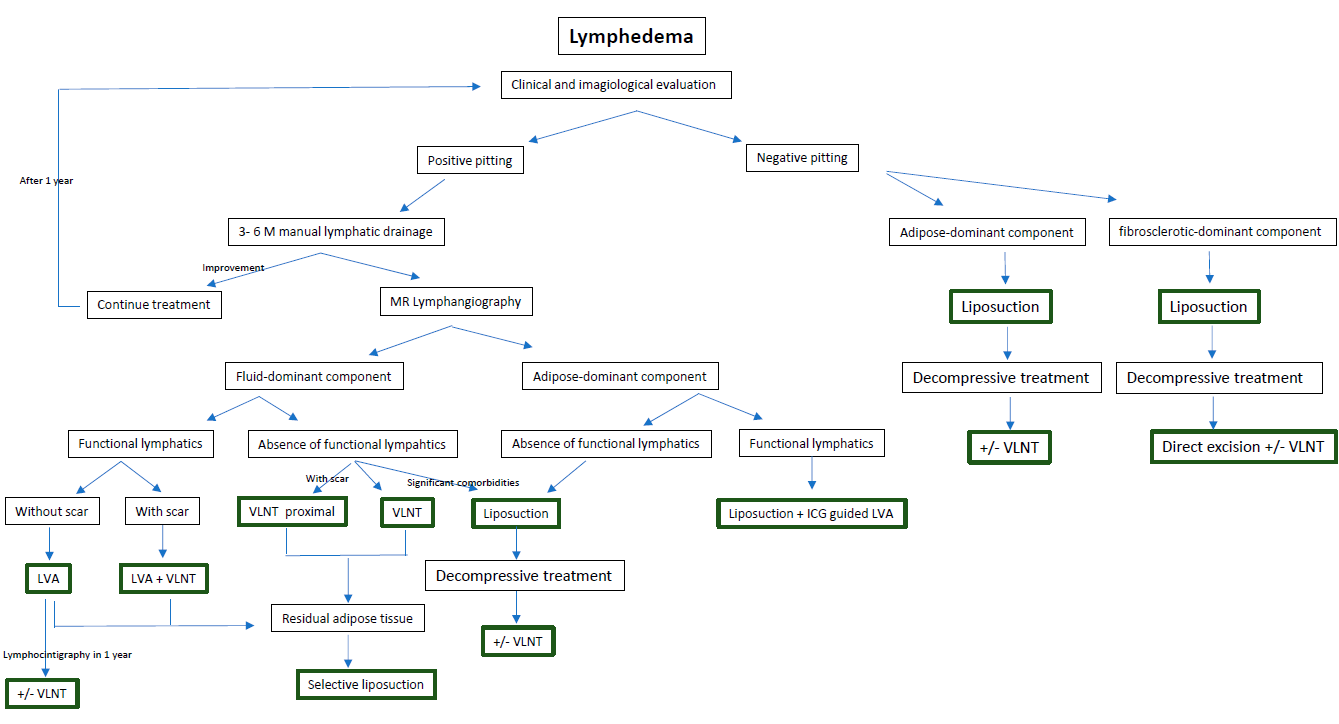
4.1. Debulking Procedures
Figure 4. A treatment algorithm for patients with symptoms of lymphedema.
4.1. Debulking Procedures
4.1.1. Suction-Assisted Lipectomy (SAL)
Suction-assisted lipectomy (SAL) is a minimally invasive surgical procedure that removes fibrotic subcutaneous adipose tissue using suction cannulas. This technique involves making small skin incisions, and power-assisted devices may be used to aid in the removal of fibrous soft tissue. The procedure is performed circumferentially from the distal end to the proximal end and in a longitudinal direction to minimize damage to the remaining lymphatics, although some damage is inevitable. The goal of SAL is to remove the maximal amount of adipose tissue, and the incisions are left open to drain externally. Intraoperatively, custom compression garments are applied, and the lifelong use of compression garments is required to prevent recurrence [25][26][25,26]. Several studies have confirmed the long-term reduction in volume, an improvement in quality of life, and a decrease in infection rates with SAL [26][27][28][26,27,28]. In selected patients, SAL may be performed in conjunction with physiologic surgery to reduce the dependence on compression garments and to improve outcomes [26]. However, SAL is not effective for patients with end-stage fibrosclerotic lymphedema, and direct excision is required in those cases.4.1.2. Direct Excision
In cases of advanced fibrosclerotic lymphedema, the direct excision of the diseased interstitial tissues may be necessary, with or without skin resection. The Charles procedure involves removing subcutaneous tissues and skin circumferentially, followed by applying skin grafts over the muscle fascia. Modified versions of this procedure incorporate negative pressure wound therapy and delayed skin grafting to improve graft take and wound recovery [13]. Alternatively, the modified Homan’s procedure [26] enables primary skin closure after performing the excision in a staged manner. These procedures are indicated for patients with irreversible fibrosclerotic lymphedema and significant symptoms, such as recurrent infections, impaired mobility, ulcerations, and malignancies. Although direct excision surgery provides consistent results with an improvement in well-being and function [29], it is associated with significant morbidity, scarring, a risk of graft loss, skin flap necrosis, lymphedema distal to the excised area, and sensory loss [1]. Additionally, patients may still need to use compression garments after surgery, and in cases where lymphedema recurs in the extremity, amputations may be necessary.4.2. Physiologic Procedures
Advancements in the treatment of lymphedema have been achieved through the implementation of microsurgery, which allows for a targeted treatment of the underlying cause of the disease. Physiological procedures have been developed to restore the lymphatic flow, with the two main techniques being lymphaticovenular anastomosis (LVA) and vascularized lymph node transfer (VLNT).4.2.1. LVA
The goal of lymphaticovenular anastomosis (LVA) is to restore lymphatic circulation by connecting functional lymphatic channels to subdermal venules of similar size, creating peripheral shunts within the lymphedematous limb. This allows for unidirectional flow from the congested high-pressure lymphatic system to the lower-pressure venous system [30]. To achieve successful long-term LVA, certain principles must be followed. Candidates for LVA must have functional or at least draining lymphatic vessels and a venule in proximity without reflux. As described before, there are many imaging modalities available to evaluate the functional status of lymphatics and, consequently, to predict the outcome of LVA. The most used modality is ICG-L. Since all of the lymphatic pathways do not deteriorate concurrently or to the same extent, early-stage patients are more likely to have functional lymphatics in superficial distribution that are easily visualized via ICG-L. On the other hand, patients in advanced stages may benefit from methods such as MRL or SPECT/CT to reveal deeper functional lymphatics. Therefore, the integration of different imaging modalities (ex., ICG-L and MRL) increases the reliability of the preoperative localization of functional lymphatics and may predict the outcome of LVA. Regarding the existence of a venule in proximity without reflux, as dynamic venous stenosis may contribute to lymphedema, some authors recommend screening for venous compression or reflux as part of the perioperative evaluation. This allows for the evaluation of the utility of scar fibrosis release, angioplasty, or venous stenting in the treatment of venous insufficiency [31][32][31,32]. A meticulous surgical technique is critical for a successful long-term LVA [26]. At selected cutaneous sites, two-centimeter skin incisions are made, and lymphatic channels and venules are carefully dissected under a microscope using super-microsurgery instruments. Nylon sutures of 11–0 or 12–0 are used for the anastomosis, and the anastomotic technique is selected depending on the relative calibers of the vessels found. Veins and lymphatics can mainly be anastomosed in the end-to-side, end-to-end, side-to-side, and side-to-end arrangements. The most efficient bypass is considered by some authors to be the side-to-end arrangement, as it allows for bidirectional lymph flow into a recipient vein while preserving the native lymph flow of the vessels and preventing damage to existing vessels [6]. However, there is no established consensus regarding the best technique to use or the number of anastomoses to perform [33][34][33,34]. After performing the anastomosis, its patency can be tested with ICG-L or patent blue dye (Figure 54). LVA is more effective in patients with early stages of lymphedema, although there can be a synergistic benefit when performed synchronously with vascularized lymph node transplantation in advanced-stage lymphedema. In fact, lymphedema treatment is a highly individualized process, and its effectiveness is consistently enhanced in combined approaches [30]. The eligibility of the LVA procedure is mainly determined by the presence of healthy, functional lymphatic vessels rather than the stage of lymphedema [32][35][32,35]. LVA can also be helpful in the treatment of lymphorrhea, as demonstrated by some studies [36][37][36,37]. The complications of LVA are minimal, with rates reported at 5.9% [30]. LVA offers significant advantages for the patient, being a minimally invasive procedure that can be performed as an outpatient procedure under local or general anesthesia, with a short recovery time and minimal postoperative restrictions [32] (Figure 65).
Figure 54.
LVA was performed successfully in an end-to-end fashion. The patent blue on the subdermal venule shows the patency of LVA.

Figure 65. (a) Patient with right upper extremity lymphedema stage 1; (b) patient treated with 3 LVA. A reduction in the interlimb volume from 34% to 12% was achieved.
4.2.2. VLNT
For later stages of lymphedema, when the lymphatic channels are obliterated, alternative surgical interventions have been proposed, including vascularized lymph node transfer (VLNT). VLNT is a microsurgical technique that involves the transplantation of a vascularized lymph node and the surrounding tissue into the affected limb, anastomosing it to the arterial and venous systems in the recipient site. Typically, VLNT is performed for patients with moderate to advanced lymphedema, with damaged lymphatic vessels or decreased lymph node function. The precise physiological mechanisms behind the effects of VLNT on lymphedema are not yet fully understood. However, two primary hypotheses have been put forward to explain these effects. The first hypothesis proposes that VLNT induces lymphangiogenesis, which establishes connections between the lymph nodes and the recipient site’s lymphatic vessels. The second hypothesis suggests that the transferred lymph node functions as a “pump” by absorbing the interstitial fluid and transporting it into the systemic circulation via the intrinsic lymphovenous shunt within the nodes [38]. The proposed mechanisms provide evidence for the efficacy of both proximal anatomical (orthotopic) and distal non-anatomical (heterotopic) placement of lymph node flaps. Indeed, there is an ongoing debate regarding the ideal location of the recipient site [33]. Typical locations for VLNT include the axilla, elbow, wrist, groin, knee, and ankle. In selected patients who have planned for postmastectomy breast reconstruction and are suitable for autologous reconstruction, a chimeric flap of deep inferior epigastric artery perforator (DIEP) and a groin vascularized lymph node flap placed in the axilla may be suggested as an optimal solution for breast reconstruction and lymphedema. Since upper-extremity lymphedema often occurs after previous surgery with or without radiation to the axilla, scar tissue in the area and around the axillary vein may need to be released to provide a healthy bed for lymphangiogenesis. Similar to the axilla, the groin region may also need extensive removal or dissection of the scar tissue from past surgeries and radiotherapy [32]. In such situations, the orthotopic placement of VLNT is likely more reasonable as it can address both objectives. However, research suggests that the selection of the recipient sites does not have a significant impact on the outcomes, and hence, the choice is typically based on the availability of recipient vessels and surgeon preference. Although multiple studies have shown encouraging results of vascularized lymph node transfer (VLNT) in improving the symptoms and quality of life of patients with lymphedema, patients are still required to use compression garments after the surgery [39]. There are several potential donor sites for VLNT, including the groin, lateral thoracic, supraclavicular, submental, omental, and jejunal mesenteric node flaps (Table 2). Among these options, the most commonly used is the groin flap [40]. Its surgical anatomy and safety of harvesting has been clearly described [41]. Although VLNT has shown promising results in treating lymphedema, mild to severe secondary iatrogenic lymphedema at the donor site was reported in some cases [1][25][40][42][1,25,40,42]. Even in the absence of clinical lymphedema of the donor site, lymphatic function alterations were seen; thus, caution should be taken [43][44][43,44]. However, according to the literature, symptomatic iatrogenic donor-site lymphedema is a rare complication [25][33][25,33]. To minimize this risk, reverse lymphatic mapping was suggested as a mandatory test, involving the injection of ICG or patent blue dye in the distal part of the limbs and the avoidance of marked draining nodes during flap harvesting [45]. Other complications, such as seroma, lymphocele, infection, and delayed wound closure, have also been observed. Compared to LVA, VLNT requires longer hospital stay and surgical time [25].Table 2.
Characteristics of the vascularized lymph node flap options.
| VLNT | Advantages | Limitations |
|---|---|---|
| Groin flap |
|
|
| Lateral thoracic flap |
|
|
| Supraclavicular flap |
|
|
| Submental flap |
|
|
| Omental flap |
|
|
| Jejunal flap |
|
|
